amides are nitrogenous organic compounds whose main characteristic is the presence of a carbonyl group (carbon that performs a double bond with oxygen), bonded directly to a nitrogen, which in turn can bond to two atoms of hydrogen.
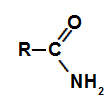
Functional group of an amide
Classification of amides
a) Simple amide
And the amide which has two hydrogens bonded to the nitrogen of the functional group.

Structural formula of a simple amide
b) Monosubstituted amide
And the amide which has only one hydrogen bonded to the nitrogen of the functional group, since the other has been replaced by an organic radical.

Structural formula of a monosubstituted amide
c) Disubstituted amide
And the amide which has no hydrogen bonded to the functional group nitrogen, as they have all been replaced by organic radicals.

Structural formula of a disubstituted amide
Nomenclature of amides
The IUPAC naming rule that should be used for a amide é:
Prefix + Infix + amide
In which:
Prefix: always related to the amount of carbon present in the chain;
Infix: always related to the type of bonds between the carbon atoms in the chain.
Example:
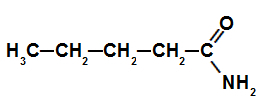
Structural formula of an amide with 5 carbon atoms
In this example, an amide contains 5 (pent prefix) carbon atoms, and only single bonds (an infix) between those carbon atoms. For that reason, its name is pentanamide.
If amide is branched, the name and position of each radical will be written before the prefix, as agreed. Note the example below:
Example: branched amide
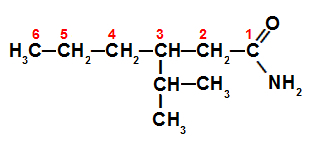
Branched amide structured for nomenclature
Do not stop now... There's more after the advertising ;)
Such as amide is branched, it is essential to determine its main chain (with the greatest number of carbons from the functional group), which is numbered above. In this case, the main chain has 6 carbons (hex prefix), only single bonds (an infix) between the carbons and an isopropyl radical on carbon 3. The name of this amide is therefore 3-isopropylhexanamide.
In monosubstituted or disubstituted amides, the position of the branch will always be indicated by N, since the radical is directly linked to nitrogen. If there is another radial in the chain, it will be written after the indication of the N's radical.
Example: substituted amide

Substituted amide structured for nomenclature
Like this amide, in addition to being substituted, is branched, it is essential to determine its main chain. Thus, we have a main chain with 6 carbons (prefix hex), only single bonds (infix an) between the carbons, a methyl radical on carbon 3 and an ethyl radical on nitrogen. Therefore, the name of this amide is N-ethyl-3-methylhexanamide.
Physical characteristics of amides
They are less dense than water;
They have a basic character;
feature polar molecules;
The intermolecular forces that hold amide molecules together are the permanent dipole;
They are found in solid state at room temperature, with the exception of methanamide, which is liquid;
They have good solubility in water when they have few carbon atoms. However, the greater the number of carbon, the lower the solubility in water, and the greater the solubility in organic solvents;
They have higher melting and boiling points when compared to other organic compounds.
Uses of amides
In general, the amides are used, for example:
In the synthesis (production) of various organic compounds;
In the manufacture of explosives;
In the production of lacquers (certain type of resin);
In the production of fertilizers;
In the production of medicines;
In the production of creams and ointments.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What are amides?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-sao-amidas.htm. Accessed on July 27, 2021.
Chemistry

Aldehydes, carbonyl compounds, carbonyl group, Main aldehydes, Ethanal, raw material in the pesticide and drug industry, Metanal, formaldehyde, plastic and resin industry.
Amines, classification of amines, amine properties, primary amine, nitrogenous organic compounds, alkyl radicals, dimethylamine, ethylamine, trimethylamine, compounds extracted from vegetables, putrescine, cadaverine, organic bases, syntheses organic


