You nitro compounds are characterized by the presence of the following functional group:
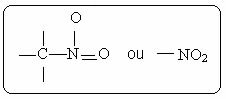
Among its derivatives, the best known is nitrobenzene, which is a yellow, toxic, water-insoluble, dense liquid used as a solvent for organic substances.
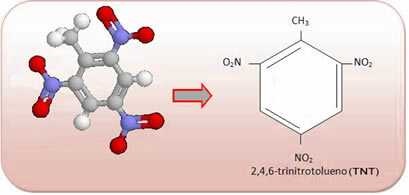
These compounds are very reactive, so they are widely used as explosives. Some well-known examples are 2-methyl-1,3,5 - trinitrobenzene or 2,4,6-trinitrotoluene (TNT), or 2,4,6 - dinitrotoluene (DNG) and trinitroglycerin (TNG), which, in addition to being an explosive, is also used as a coronary vasodilator in case of risk of infarction.
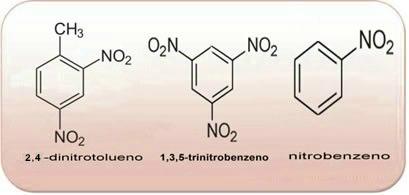
The above compounds are all aromatic, the greater the amount of NO2 in its molecules, the more explosive it will be. Their preparations and properties are very different from those of aliphatic nitro compounds. Nitroalkanes are colorless, polar liquids, also insoluble in water and used in organic syntheses, as intermediates and as solvents.
The nomenclature of nitrocompounds follows the rule below, established by the International Union of Pure and Applied Chemistry (IUPAC):

Thus, we have the following names for the nitrocompound structures below:
H3Ç__AT THE2: nitromethane
H3Ç__CH2__AT THE2: nitroethane
H3Ç__CH2__ CH2__AT THE2: 1- nitropropane
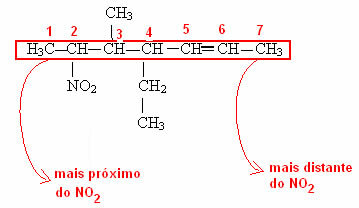
Note in the last example that it was necessary to number where the functional group of the carbon chain is coming from. This numbering is always done starting at the closest end of the functional group. See how this is done in the example below:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nitrocompostos.htm
