Among the intermolecular forces, the induced dipole-induced dipole forces they are the only ones not studied by the Dutch physicist Johannes Diederik Van der Waals (1837-1923). They were elucidated by the German physicist Fritz Wolfgang London (1900-1954), so these forces are also called london forces or london dispersion forces. Another name given to these forces is instantaneous dipole-induced dipole.
This kind of force occurs in non-polar substances, such as H2, O2, F2, Cl2, CO2, CH4 and C2H6, among others. And they can also occur between noble gas atoms, when they approach, causing repulsion between their electrospheres. In this way, the electrons accumulate on a certain side, which is negatively polarized and the opposite side positively, due to the negative charge deficiency.
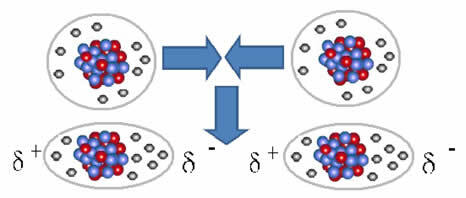
Nonpolar molecules can go from a gaseous state – in which they are very far apart and there is no interaction, as there are no poles – to a liquid and solid state. In these states of aggregation, the molecules are closer and the electronic attractions or repulsions between their electrons and nuclei can lead to a deformation of their electronic clouds, momentarily, originating positive and negative poles temporary.
Instantaneous dipoles can induce polarization of neighboring molecules, resulting in attractive forces.
This induction can also occur. between different molecules and generally these forces are weaker in intensity than the dipole-dipole and hydrogen bond strengths. Therefore, solids with this interaction strength such as dry ice (carbon dioxide - CO2) and iodine (I2), which are in the solid state, sublime (go to the gaseous state); because the energy needed to disrupt their interactions is small.
An example of intermolecular forces between polar and nonpolar molecules occurs between oxygen gas (nonpolar) and water (polar). It turns out that the negative end of the water approaches the O2, repelling itself, and thus the electronic cloud of the non-polar molecule moves away. The oxygen is then momentarily polarized and starts to interact with the water, solubilizing in it.
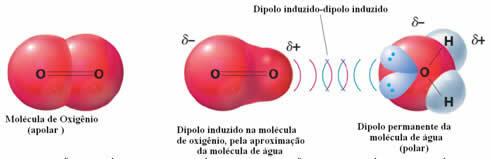
Since these forces are weak, the solubility of this gas in water is small. Even so, its presence is essential to preserve the life of various aquatic organisms.
This interaction force also occurs in nature, providing adherence between the geckos' paws and the surface on which they walk. That's why they can walk on walls and ceilings without falling or sticking.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/forcas-dipolo-induzido-dipolo-induzido-ou-dispersao-london.htm