In order to reverse the increase in overweight and obesity among young people aged 5 to 19 years, the Federal Government has been promoting measures aimed at promoting healthy eating in Brazil. Among these measures, the food given in school canteens and the eating habits of these young people, who are used to consuming processed foods with a lot of fat, stand out.
In the portal of Health Department shows that from the population aged 10 to 19 in Brazil, 34% consume foods with high fat content, which causes an increase in obesity and a higher incidence of cardiovascular diseases.
Fats are essential macronutrients for the composition of our metabolism, which must make up 15% to 30% of our diet, respecting the specifics of each metabolism and level of physical activity. They are important for the body because the fatty acids obtained from the digestion of fat are fundamental components of the phospholipids, which make up cell membranes. Furthermore, fats are responsible for transporting several vitamins, such as A, E, D and K.
Nonetheless, the fat has a high caloric content, being around 9 kcal/g. Therefore, its excessive intake causes health problems. For example, saturated fatty acids, and more recently trans fats, have been identified as possible. cause of the aggravation of cardiovascular diseases due to increased levels of low-density lipoprotein (LDL) in the blood. Scientific research has also associated the fact of eating more trans fats with a higher incidence of myocardial infarctions and strokes. Therefore, on food labels, in addition to the total fat content, they must also be mentioned in terms of saturated fat and trans fats. But what is the difference between them?
To understand, let's look at the chemical makeup of fats:
Fats are predominantly derived from saturated fatty acids. The only difference between an oil and a fat is that oils are unsaturated and become liquid at room temperature. Fats, on the other hand, are saturated (they only have simple bonds in their carbon chains) and appear in the solid phase under ambient conditions.
Fat is of the class of lipids, that is, esters that when reacting with water form a fatty acid and a fatty monoalcohol or a polyalcohol (glycerin) and, eventually, other compounds. You lipidsare classified into four groups, one of which is glycerides. Glycerides, on the other hand, are triesters formed from three fatty acid molecules and a glycerin trialcohol molecule.
It is in this group that fats are found, as they have as main components the triacylglycerols (ester formed from glycerol (alcohol) and three fatty acid molecules (naturally occurring carboxylic acids) in a process catalyzed by enzymes (lipases) or acidic medium).
The figure below shows a generic chemical reaction to form a triacylglycerol:

Triacylglycerol formation reaction.
The fatty acids that form fats and oils can be saturated or unsaturated. Thus, fats that are made up of saturated fatty acids are called saturated fats.. These fats rich in saturated fatty acid chains have a tendency to solidify at low temperatures. Some examples of sources of this type of fat are butter, pork tallow, sirloin fat, coconut fat and cocoa butter.

Foods that have saturated fat in their constitution
Saturated fats are normally prepared through a hydrogenation reaction (addition of hydrogen) to vegetable oils, in the presence of a nickel or platinum catalyst at close temperatures at 100°C. See below an example of this type of reaction, in which the unsaturation (double bond) was broken and each of the atoms involved bonded to a hydrogen atom of the reactant substance:
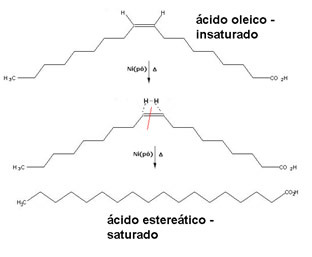
Hydrogenation of oleic acid
With partial hydrogenation, that is, with insufficient hydrogen to hydrogenate all unsaturations existing, an isomerization reaction occurs with formation of trans fatty acids, from chains unsaturated. One example is hydrogenated vegetable fat, which is obtained by this method, resulting in a mixture of cis and trans saturated and unsaturated fat.
Thus, trans fats have unsaturated fatty acids in their composition with one or more trans-type double bonds.
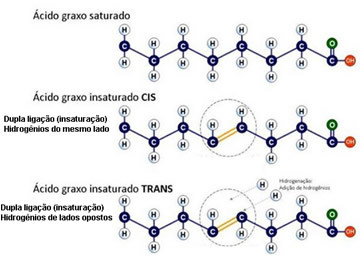
Saturated and unsaturated fatty acid, cis and trans
Thus, this substance is unusual in nature and is present in industrialized foods, such as: ice cream, diet chocolates, chocolate bars, salty snacks package, industrialized cakes/pies, biscuits, cream biscuits, commercial fries, ready-to-eat sauces, puff pastries, pastries, mayonnaise, candied sugar topping, microwave popcorn, canned soups, margarines, vegetable creams, hydrogenated vegetable fats, breads and bakery products and potatoes fries.

Foods that have trans fat in their constitution
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/saude-na-escola/composicao-quimica-das-gorduras.htm
