You basic oxides they are those that, when reacting with water, give rise to bases. When they react with an acid, they form salt and water.
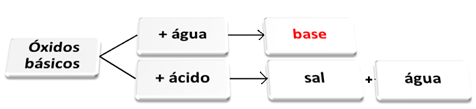
Basic oxides reacting with water and acid.
For example, we have sodium oxide (Na2O), which is a basic oxide. See below that, when reacting with water, it forms sodium hydroxide base:
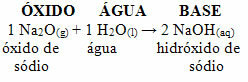
If that same oxide reacts with an acid such as sulfuric acid (H2ONLY4), a salt (sodium sulfate) and water will be produced.
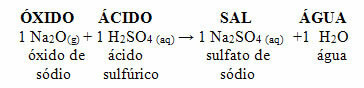
If a basic oxide reacts with an acidic oxide, a salt is also produced, but without the water.
As shown in the example shown, the other basic oxides are also ionic compounds, being formed in their mostly by metals, elements that are highly electropositive, normally presenting a "charge" equal to +1 or +2.
Examples:
At2O = Na1+
K2O = K1+
CaO = Ca2+
MgO = Mg2+
These compounds have high melting and boiling points and all enclose the oxygen anion (O2-).
Applications of some basic oxides:
• Dog: Calcium oxide
This compound is commonly known as quicklime. When reacting with water, as per the reaction below, a base (calcium hydroxide) is formed which is used to paint walls, tree trunks and other materials, with the main purpose of repelling insects, preserving the seal and preventing infiltration from water. This formed base is called
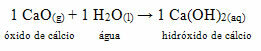

Whitewash painting.
• K2O: potassium oxide
Potassium oxide is commonly found in wood and cigarette ash. When reacting with water, it forms the base potassium hydroxide (KOH), used in the manufacture of soaps. Even in the past, in places where there was no KOH, vegetable ashes were used, which contained K2O, to react with fats and produce the so-called "grey soap".
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidos-basicos.htm
