O heat is the thermal energy in transit between two bodies, motivated by the difference in temperature. This energy always flows from the body with the highest temperature to the body with the lowest temperature, and its flow only ceases when the temperature of the bodies involved becomes equal.
Types of heat
When exposed to a thermal source, any material can change its temperature or change its temperature. physical state of aggregation of the molecules.
heat is said sensitive when it is only able to change the temperature of a body. heat is said latent when it changes the temperature and generates change in the state of aggregation of the molecules of a substance.
Calculation of latent heat
the amount of latent heat is determined by the product of the mass (m) of the body that underwent the state transformation and the so-called latent heat of fusion or vaporization (L).
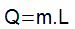
The amount of heat needed for each gram of substance to change state is called the latent heat of vaporization or fusion. In the case of water, for example, the latent heat of vaporization is 540 cal/g, that is, each gram of water at 100°C needs 540 cal of heat to undergo the change from liquid state to state gaseous.
Examples
1st Example (Uerj) - The graph below indicates the thermal behavior of 10 g of a substance that, when receiving heat from a source, completely passes from the solid to the liquid phase.

The latent heat of fusion of this substance, in cal/g, is equal to:
a) 70
b) 80
c) 90
d) 100
Answer: Letter A
The horizontal part of the graph represents the change in physical state in which heat ceases to act in the temperature variation and starts to act in such a way as to change the aggregation state of the element. In this part of the graph, the amount of heat involved was 700 cal (1000 – 300 = 700 cal).
The latent heat of fusion can be determined from the latent heat equation:
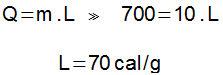
2nd Example - A mass of 2000 g of water is exactly at 100°C. Knowing that the heat of vaporization of water is 540 cal/g, determine the amount of heat, in kcal, needed to vaporize 30% of the mass of water.
a) 224
b) 250
c) 300
d) 360
e) 324
Answer: Letter E
The mass of water to be considered is 600 g, which corresponds to 30% of the total water. Thus, the amount of heat needed for vaporization will be:

By Joab Silas
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/fisica/o-que-e-calor-latente.htm