Sulphates are ionic compounds that contain the anion SO42-, which is called the sulfate anion.
Sulfur is an element of the 16 or VI-A family, which means it has 6 electrons in its valence shell. According to the octet rule, it would need to receive 2 more electrons to have 8 electrons in the last electron shell and thus be stable. But sulfur undergoes octet expansion, as it is an element located in the third period of the Table Periodic, being relatively large, which allows it to accommodate more than eight electrons to its around.
Thus, as can be seen below, the valence level of sulfur expands to house a total of 12 electrons. But two oxygen atoms are still not stable, each with seven electrons in the valence shell, thus needing to receive one more electron to stabilize. For this reason, the charge of each of these two atoms is -1, resulting in a total charge for the anion of -2:
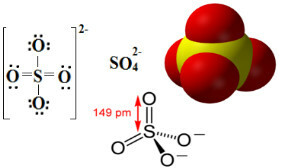
The sulfate anion can come from sulfuric acid (H2ONLY4(aq)) shown below:
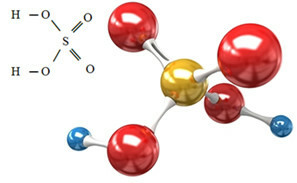
Sulfuric acid reacts with some base, in a neutralization reaction, forming an inorganic salt, which is our sulfate, and also water:
Generic reaction: Sulfuric Acid + Base → Salt (sulfate) + Water
H2ONLY4 + 2 Çoh →Ç2ONLY4+ 2 H2O
See an example below where potassium sulfate is formed:
H2ONLY4 + 2 KOH →K2ONLY4+ 2 H2O
Note that the nomenclature of a sulfate is done simply by the rule: sulfate + name of the element attached to it. In addition, the formulas for sulfates are made by exchanging the charge of each ion for the element index, with the index “1” not needing to be written:
Examples:
At+1 ONLY42- → At2ONLY4→ sodium sulfate
mg+2 ONLY42- → MgSO44→ magnesium sulfate
Here+2 ONLY42- → Case4→ calcium sulfate
Ba+2 ONLY42- → BASO4→ barium sulphate
Al+3 ONLY42- → Al2(ONLY4)3→ aluminum sulfate
Salts containing the sulfate ion are usually soluble in water, the exceptions being four: calcium sulfate (CaSO4), strontium sulfate (SrSO4), barium sulfate (BaSO4) and lead sulfate (PbSO4). This is because there is a high binding energy between these cations (Ca2+, sir2+, Ba2+ and Pb2+) and the sulfate, since they all have a +2 charge and the sulfate anion has a -2 charge. Thus, severing that connection becomes more difficult.
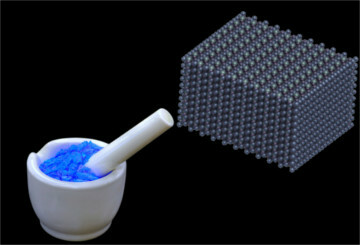
Sulphates are found in the form of crystalline lattices, due to the attraction that opposite charges exert on each other, as shown below in the case of copper sulfate (CuSO4):
Sulphates are found in nature mainly in the form of minerals. See some examples:
barite → barium sulphate;
Gypsum → calcium sulfate;
Celestite → strontium sulfate;
anglesiOK → lead sulfate;
Glauberite → double calcium and sodium sulfate: CaNa2(ONLY4)2;
Potassium alum (kalinite) → hydrated double potassium aluminum sulfate: KAl (SO4)2.11H2O
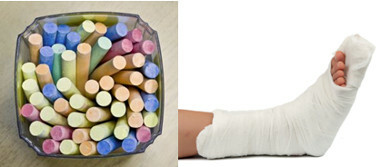
Among the main sulfates in everyday life, we have calcium sulfate, which in anhydrous form (without water) is used in the manufacture of school chalk. When it is in dihydrate form, (CaSO4 .2h2O), it is known as gypsum and is more abundant. Upon heating, it forms calcium sulfate hemihydrate (CaSO4. ½H2O), which is plaster, used in orthopedics, dental molds, civil construction and paints.
Another important salt is the smagnesium sulphate (MgSO44), known as sbitter al or Epsom salt, which has a laxative action and is used for massage and relaxing baths.
By Jennifer Fogaça
Graduated in Chemistry

