THE propanone is the most commercially used ketone and is more commonly known as acetone, being widely used as a nail polish remover. However, the solution that serves this purpose is actually a mixture of acetone, ethyl alcohol and water.
Acetone is relatively toxic and can attack the oral and nasal mucosa and cause skin irritation.
Isolated propanone has the following structure:

At room temperature, acetone is a flammable, colorless, pleasant-smelling liquid, soluble in water and other organic solvents. This last feature causes acetone to be used mainly as a solvent not only for enamels, but for paints, varnishes, fiberglass, in the extraction of fats and oils from vegetable seeds (such as soy, peanuts and sunflower) and in industry food. It is also used in the production of acetic anhydride, in the preparation of chloroform, iodoform and bromoform, in the production of medicines and as a celluloid solvent.

She can be industrially obtained by thermal decomposition of calcium acetate, by hydration of propene or by oxidation of cumene. In the latter case, cumene is obtained through a reaction between propene (propylene) and benzene, derived from petroleum.
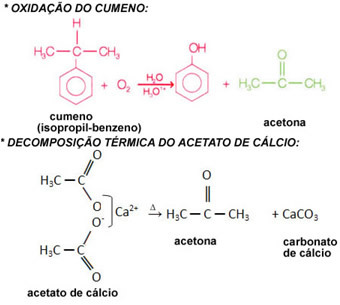
Unfortunately, propanone is also used in extracting cocaine from coca leaves; therefore, it is marketed in a controlled manner by the Federal Police's Narcotics Department.
Our bodies produce propanone as a result of incomplete breakdown of fats. The normal level of propanone in the blood is up to 1 mg/100 mL of blood. However, in some cases of illnesses such as diabetes Millitus and hyperthyroidism, the person produces more propanone that can be detected in the urine, where it is excreted, and that can even be felt on the breath.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/composicao-quimica-propanona-acetona.htm
