Thermodynamics is an area of physics that studies energy transfers. It seeks to understand the relationships between heat, energy and work, analyzing amounts of heat exchanged and the work performed in a physical process.
Thermodynamic science was initially developed by researchers who were looking for a way to improve machines, in the period of the Industrial Revolution, improving their efficiency.
This knowledge is currently applied in various situations of our daily lives. For example: thermal machines and refrigerators, car engines and processes for transforming minerals and petroleum products.
Laws of Thermodynamics
The fundamental laws of thermodynamics govern how heat becomes work and vice versa.
First Law of Thermodynamics
THE First Law of Thermodynamics relates to the principle of energy conservation. This means that energy in a system cannot be destroyed or created, only transformed.
The formula that represents the first law of thermodynamics is as follows:
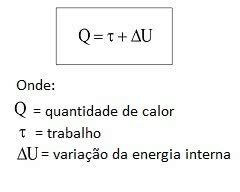
The amount of heat, work and variation of internal energy have as standard unit of measure the Joule (J).
A practical example of energy conservation is when a person uses a pump to inflate an inflatable object, he is using force to pump air into the object. This means that kinetic energy makes the piston go down. However, part of this energy is transformed into heat, which is lost to the environment.
THE Hess' Law is a particular case of the principle of energy conservation. Know more!
Second Law of Thermodynamics
At heat transfers they always occur from the warmest body to the coldest body, it happens spontaneously, but not the other way around. Which is to say that thermal energy transfer processes are irreversible.
In this way, by the Second Law of Thermodynamics, it is not possible for heat to be fully converted into another form of energy. For this reason, heat is considered a degraded form of energy.

The physical quantity related to the Second Law of Thermodynamics is the entropy, which corresponds to the degree of disorder of a system.
Read too:
- Carnot Cycle
- Thermal expansion
Zero Law of Thermodynamics
THE Zero Law of Thermodynamics deals with the conditions for obtaining the thermal balance. Among these conditions we can mention the influence of materials that make the thermal conductivity higher or lower.
According to this law,
- if a body A is in thermal equilibrium in contact with a body B and
- if this body A is in thermal equilibrium in contact with a body C, then
- B is in thermal equilibrium in contact with C.
When two bodies with different temperatures are brought into contact, the one that is warmer will transfer heat to the one that is cooler. This causes temperatures to equalize reaching the thermal balance.
It is called the zero law because its understanding proved necessary for the first two laws that already existed, the first and second laws of thermodynamics.
Third Law of Thermodynamics
THE Third Law of Thermodynamics it appears as an attempt to establish an absolute reference point that determines entropy. Entropy is actually the basis of the Second Law of Thermodynamics.
Walther Nernst, the physicist who proposed it, concluded that it was not possible for a pure substance with a temperature of zero to have entropy at an approximate value of zero.
For this reason, it is a controversial law, considered by many physicists as a rule and not a law.
thermodynamic systems
In a thermodynamic system there can be one or several bodies that are related. The environment that surrounds it and the Universe represent the environment external to the system. The system can be defined as: open, closed or isolated.
 thermodynamic systems
thermodynamic systems
When the system is opened, there is a transfer of mass and energy between the system and the external environment. In the closed system there is only energy transfer (heat), and when it is isolated there is no exchange.
behavior of gases
The microscopic behavior of gases is more easily described and interpreted than in other physical states (liquid and solid). That's why gases are used the most in these studies.
In thermodynamic studies ideal or perfect gases are used. It is a model in which particles move chaotically and interact only in collisions. Furthermore, it is considered that these collisions between the particles, and between them and the walls of the container, are elastic and last for a very short time.
In a closed system, the ideal gas presupposes a behavior that involves the following physical quantities: pressure, volume and temperature. These variables define the thermodynamic state of a gas.
 Behavior of gases according to gas laws
Behavior of gases according to gas laws
Pressure (p) is produced by the movement of gas particles inside the container. The space occupied by the gas inside the container is the volume (v). And the temperature (t) is related to the average kinetic energy of the moving gas particles.
Read too Gas Law and Study of Gases.
internal energy
The internal energy of a system is a physical quantity that helps to measure how the transformations a gas undergoes occur. This magnitude is related to the variation in temperature and kinetic energy of particles.
An ideal gas, made up of just one type of atom, has internal energy directly proportional to the temperature of the gas. This is represented by the following formula:

Solved Exercises on Thermodynamics
question 1
A cylinder with a movable piston contains a gas at a pressure of 4.0.104N/m2. When 6 kJ of heat is supplied to the system, at constant pressure, the gas volume expands by 1.0.10-1m3. Determine the work done and the change in internal energy in this situation.
Correct answer: the work done is 4000 J and the internal energy change is 2000 J.
Data:
P = 4,0.104 N/m2
Q = 6KJ or 6000J
ΔV = 1,0.10-1 m3
T =? ΔU = ?
1st Step: Calculate the work with the problem data.
T = P. ΔV
T = 4.0.104. 1,0.10-1
T = 4000 J
2nd Step: Calculate the variation of the internal energy with the new data.
Q = T + ΔU
ΔU = Q - T
ΔU = 6000 - 4000
ΔU = 2000J
Therefore, the work performed is 4000 J and the internal energy change is 2000 J.
question 2
(Adapted from ENEM 2011) A motor can only perform work if it receives an amount of energy from another system. In this case, the energy stored in the fuel is, in part, released during combustion so that the appliance can function. When the engine runs, some of the energy converted or transformed in combustion cannot be used to do work. This means that there is energy leakage in another form.
According to the text, the energy transformations that occur during engine operation are due to:
a) heat release inside the engine is impossible.
b) work performed by the engine is uncontrollable.
c) full conversion of heat to work is impossible.
d) transformation of thermal energy into kinetics is impossible.
e) potential energy use of the fuel is uncontrollable.
Correct alternative: c) full conversion of heat to work is impossible.
As seen earlier, heat cannot be fully converted into work. During the motor operation, part of the thermal energy is lost, being transferred to the external environment.
See too: Exercises on Thermodynamics


