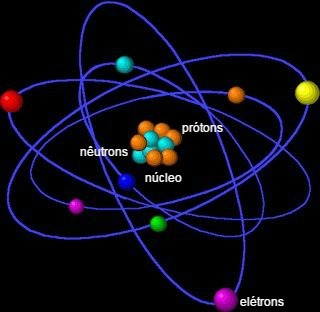
The atom is the fundamental unit of matter and the smallest fraction capable of identifying a chemical element, since it holds its identity. The term atom derives from the Greek and means indivisible.
It consists of a nucleus, which contains neutrons and protons, and electrons that surround the nucleus.
atom structure
The atom is formed by small particles, also called subatomic particless: electrons, protons and neutrons.
Most of the atom's mass is concentrated in the nucleus, a small, dense region. Its largest volume is found in the electrosphere, a place of empty spaces, as electrons orbit around the nucleus.
electrons
O electron it has a negative electric charge (-1) and almost no mass, as its value of 9.11 x 10-28 g and is about 1840 times smaller than the core mass. They are tiny particles that spin very quickly around the atomic nucleus.
The electrons that are found in the outermost regions of the atom are responsible for the formation of chemical bonds, which occur through the donation, reception or sharing of electrons.
protons
O proton it has a positive electric charge (+1) of the same absolute value as the charge on the electrons. In this way, a proton and an electron tend to attract each other electrically.
Through protons it is possible to distinguish the chemical elements, as each atom of an element has a defined number of protons in its nucleus, which is called atomic number.
neutrons
O neutron it has no charge at all, that is, it is electrically neutral. Together with the protons, it forms the atomic nucleus, which carries the entire mass of the atom (99.9%). Both the proton and the neutron have approximately the mass of 1.67 x 10-24 g. This value represents a 1 μ atomic mass unit.
The neutron provides stability to the atomic nucleus, as the nuclear force causes it to be attracted to electrons and protons.
Only the hydrogen atom has no neutrons, being made up of just an electron revolving around a proton.
Check the table below for a abstract with information about subatomic particles.
| Particle | Symbol |
Pasta (in unit of atomic mass) |
Charge (in unit of electric charge - u.c.e) |
Location |
|---|---|---|---|---|
| Proton | +1 | core | ||
| Neutron | 0 | core | ||
| Electron | -1 | electrosphere |
An atom in its ground state is electrically neutral, since the number of protons is equal to the number of electrons and the opposite charges, positive and negative, cancel each other out.
For example, sodium (Na) has atomic number 11, that is, its nucleus has 11 protons. Consequently, there are 11 electrons in the electrosphere of an atom of that element.
Read more about atomic structure.
atom composition
As we have seen, the atom is formed by a small and dense central region called the nucleus and around it there is a electrosphere, where the electrons are located, which can be divided into electronic layers, energy sublevels and atomic orbitals.
electronic layers
the atom presents energy levels, which correspond to seven layers around a nucleus and in them are the electrons that orbit around it. The layers are called K, L, M, N, O, P and Q.
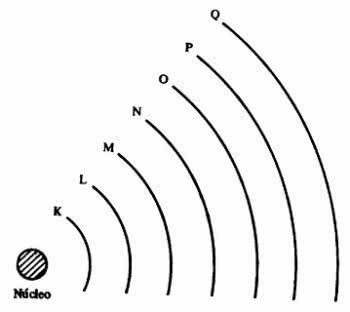
Each shell can contain a certain number of electrons, as shown in the table below.
| Energy level | electronic layer | Maximum number of electrons |
|---|---|---|
| 1º | K | 2 |
| 2º | L | 8 |
| 3º | M | 18 |
| 4º | N | 32 |
| 5º | O | 32 |
| 6º | P | 18 |
| 7º | Q | 8 |
For example, the atom of helium (He) has atomic number 2 and therefore has 2 protons in the nucleus. Consequently, in the atom's electrosphere there are only 2 electrons, which are located in the atom's first and only electronic shell, the K shell that corresponds to the first energy level.
Energy sublevels
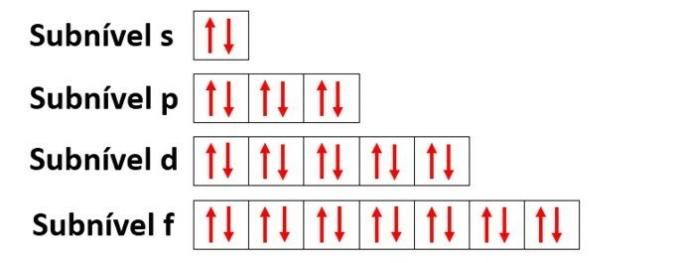
Energy levels house sublevels, which are represented by s, p, d, f. Each sublevel accommodates a maximum number of electrons, which are respectively 2, 6, 10 and 14.

With this information, it is possible to eletronic distribution of an atom and know the location of the outermost and most energetic electron.
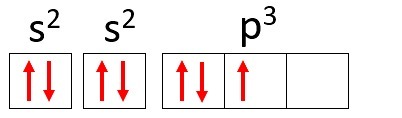
Example: Nitrogen (N)
Atomic Number: 7
Electronic distribution: 1s2 2s2 2p3
The nitrogen atom has two energy levels, K and L, and its 7 electrons occupy the s and p sublevels.
K: s2 = 2 electrons
L: s2 + p3 = 5 electrons
Note that the L shell can contain up to 8 electrons, but in the nitrogen atom there are only 5 electrons in that shell.
atomic orbitals
Orbitals characterize the region most likely to find an electron within an energetic sublevel (s, p, d, f) in an electronic shell (K, L, M, N, O, P, Q).
- s sublevel: has 1 orbital that holds up to 2 electrons
- p sublevel: has 3 orbitals that house up to 6 electrons
- Sublevel d: has 5 orbitals that house up to 10 electrons
- Sublevel f: has 7 orbitals that house up to 14 electrons
Using nitrogen again as an example and distributing its 7 electrons in atomic orbitals we would have:
Atom types
Observing the number of protons, neutrons and electrons we can compare atoms and classify them into isotopes, isobars and isotones.
A chemical element can be defined as a group of atoms with the same number of protons. These atoms are called isotopes because they have the same atomic number and different masses.
For example, in nature there are 3 isotopes of the element hydrogen (H): protium , deuterium
and tritium
.
Atoms of different chemical elements can be classified as isotones when they have different atomic numbers and masses, but the same number of neutrons.
Isobars are atoms of different elements, that is, they have a different atomic number, but the same mass number.
Read more about isotopes, isobars and isotones.
Atom models (atomic models)
The Greek philosopher Aristotle (384 a. Ç. - 322 a. C) tried to explain the constitution of all substances from the elements earth, air, fire and water.
Democritus (546 a. C - 460 a. C), a Greek scientist and mathematician, formulated the idea that there was a limit to the smallness of particles. He said they would become so small that they could no longer be divided. He called this particle an “atom”.
For most of the 19th century, it was the Dalton Atomic Model, English scientist, who proposed the atomic theory, which went far beyond the thought of the ancients.
This theory said that all substances are made up of small indivisible particles called atoms, which would be like billiard balls. As studies on the structure of matter advanced, it was discovered that the atom is formed by other small particles called subatomic particles.
With the discovery of the electron, thomson he formulated the model known as mass pudding, which described the atom as a positive sphere with negatively charged electrons embedded in its surface.
Through experiments the physicist Rutherford he found that the atom had voids and electrons around an extremely small, positive nucleus. Thus, Rutherford proposed the nuclear model to represent the atom.
Bohr improved the model proposed by Rutherford by finding that electrons do not rotate around the nucleus randomly, but in specific orbits. This model became known as the planetarium.
Also read about:
- Atomic models
- Thomson Atomic Model
- Bohr's atomic model
- Rutherford Atomic Model
- Evolution of atomic models