Electrolysis is a physical-chemical process that uses electrical energy from any source (such as battery or battery) to force the occurrence of a chemical reaction to produce simple or composite substances that cannot be found in nature or that are not found in large quantities.
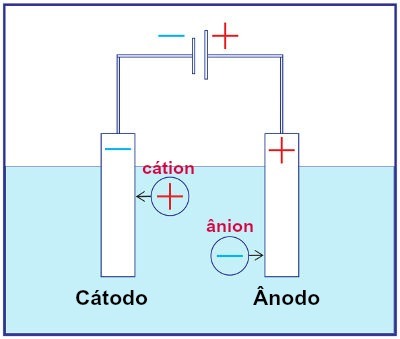
Representation of the assembly of any electrolysis system
During electrolysis, a cation undergoes reduction at the cathode, and an anion undergoes oxidation at the anode. This happens through electrical discharge provided by an external source. Thus, in electrolysis, we have a non-spontaneous oxidation and reduction reaction.
Now understand the two ways in which electrolysis occurs:
In this type of electrolysis, we use a ionic substance in the liquid state in an electrolytic vat. When the ionic substance (XY) undergoes fusion, it undergoes the process of dissociation, as represented below:
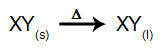

Then, when the power source is turned on, the cation (X+) moves towards the cathode, and the anions (Y-) move towards the anode. Thereby:
At the cathode: the cations receive electrons (they undergo reduction) and transform into a stable substance (X), a process represented by the following equation:
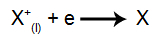
At the anode: anions lose electrons (they undergo oxidation) and become a stable substance (X), a process represented by the following equation:

a) Example of igneous electrolysis
As an example, follow now the igneous electrolysis of the sodium chloride (NaCl). When sodium chloride (NaCl) undergoes fusion, it undergoes the dissociation process, as shown below:
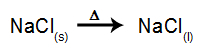
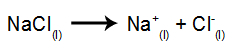
Then, when the power source is turned on, the cation (Na+) moves towards the cathode, and the anions (Cl-) move towards the anode. Thereby:
At the cathode: the cations in+ they receive electrons (they undergo reduction) and become a stable substance (Na, which is a solid metal), a process represented by the equation below:

At the anode: the Cl anions- they lose electrons (they undergo oxidation) and become a stable substance (Cl2, which is gaseous), process represented by the equation below:

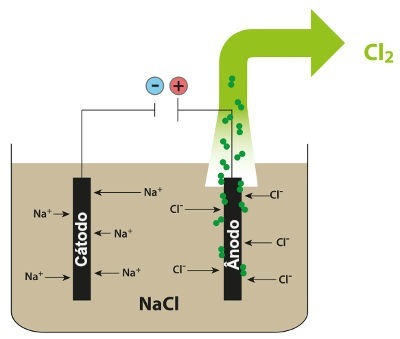
Scheme demonstrating the igneous electrolysis of NaCl
Thus, in the igneous electrolysis of sodium chloride, we have the formation of metallic sodium (Na) and chlorine gas (Cl2).
In this type of electrolysis, we use an ionic substance dissolved in water, inside the electrolytic tank. So, before performing the electrolysis, we first mix the substance (usually a salt inorganic) in water to cause its dissociation (release of a cation and an anion), as shown bellow:
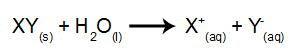
The difference in relation to igneous electrolysis is that, in addition to the ions from dissociation, we also have ions from the self-ionization of water. In its self-ionization, water produces a hydronium cation (H+) and a hydroxide anion (OH-), as in the equation below:
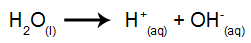
Thus, inside the electrolytic vat, we have the presence of two cations (one from the substance ionic and one from water) and two anions (one from the ionic substance and the other from the Water).
To know which cation will move to the cathode and which anion will move to the anode, it is necessary to know the order of discharge of cations and anions.
For cations:
Au>Pt>Hg>Ag>Cu>Ni>Cd>Pb>Fe>Zn>Mn>hydronium>IIIA family> IIA family > IA family
for anions
Non-oxygenated anions and HSO4 > hydroxide > oxygenated anions and F
Then, when the power source is turned on, a cation (X+) moves towards the cathode, and one of the anions (Y-) moves towards the anode.
At the cathode: the cations receive electrons (they undergo reduction) and transform into a stable substance (X), a process represented by the equation below:

At the anode: anions lose electrons (they undergo oxidation) and turn into a stable substance (Y), a process represented by the equation below:
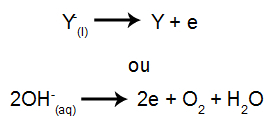
a) Example of aqueous electrolysis
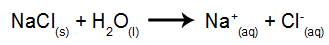
As an example, we will use aqueous electrolysis of sodium chloride (NaCl). When sodium chloride (NaCl) is dissolved in water, it undergoes the dissociation process, as shown below:
In addition to the dissociation of NaCl, we have the self-ionization of water:
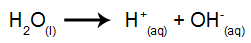
So we have the H cations+ and on+ and the OH anions- and Cl-. Then, when the power source is turned on, we have the following:
at cathode: the H cations+ receive electrons (reduced) and become a stable substance (H2, which is a gas). This is because hydronium has discharge priority over the elements of the IA family (in this case, Na). The process is represented by the equation below:
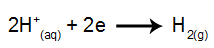
at anode: the Cl anions- they lose electrons (they undergo oxidation) and become a stable substance (Cl2, which is gaseous). This is because the Cl- it is a non-oxygenated anion and has discharge priority over hydroxide, a process represented by the equation below:


Scheme demonstrating the aqueous electrolysis of NaCl
Thus, in the aqueous electrolysis of sodium chloride, we have the formation of hydrogen gas (H2) and chlorine gas (Cl2).
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-eletrolise.htm

