Substances are materials that have all properties well defined, determined and practically constant.
See three examples:
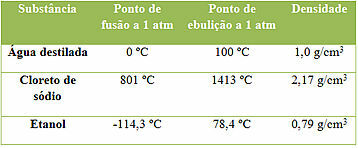
Examples of three substances and their properties
Note that these are three substances and that their properties are constant when measured under the same temperature and pressure conditions. But each has different property values, as no two substances have all properties exactly the same. This is important because they can be used to identify a substance.
Atoms are the smallest constituent parts of matter, and they can remain isolated, as occurs, for example, in the case of helium gas, which is formed by atoms of helium (He); but they can also bind in various ways, forming molecules (in the case of those that bind covalently by sharing pairs of electrons) or ionic clusters (which bind by ionic bonding, in which one definitively donates electrons to the other and ions are formed that form attract).
Thus, substances can be formed by atoms, molecules or ionic clusters. Water and ethanol are formed by molecules, which are, respectively, H
2O and C2H5OH, while sodium chloride is formed by ionic agglomerates, whose unitary formula is NaC?.
Water and salt are examples of substances
These examples show that substances can be classified according to the grouping of atoms that form them. If the atoms that join to form the substance are of the same chemical element, then we have a simple substance. Some examples are: oxygen gas (O2), hydrogen gas (H2), iron (Fe), helium gas (He), aluminum (A?) etc.
On the other hand, if substances are formed by atoms of two or more different elements, such as water, ethanol and sodium chloride, then they will be classified as a compound substance.
In nature it is not very common to find substances in an isolated, pure form. They are usually found mixed with other substances. For example, the mineral water we drink contains many dissolved substances, not just H molecules.2O; that's why she is a Mix, and not a substance.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-uma-substancia.htm


