Cells or batteries that have lithium as their main constituent have as one of their characteristics the fact that they are very light, as lithium is the least dense metal discovered so far. To give you an idea, this silvery white metal floats in the water, as it is twice as dense as it is. This is due to the fact that lithium has only three protons and three neutrons.
There are two main types of lithium batteries, one of which is called lithium iodine battery. It was primarily developed for use in cardiac pacemakers, since it is very light, safe (does not release gases, as it is hermetically closed), it has a good durability (approximately 8 to 10 years), provides a voltage of 2.8 V and a high charge density (0.8 Wh/cm3).
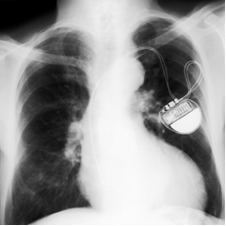
The electrodes are formed by lithium and an iodine complex, which are separated by a crystalline layer of lithium iodide that allows the passage of electric current. O metallic lithium works like the anode of this cell, that is, it is the negative pole that oxidizes, losing electrons. already the
cathode, the positive pole that reduces, receiving electrons, is the iodine complex.See the semi-reactions that occur in the electrodes and the equation that represents the global reaction of this type of cell:
Anode Half Reaction: 2 Li(s) →2 I read+(s) + 2e-
Cathode Semi-Reaction: 1 I2(s) + 2e-→2 I-(s)
Global Reaction: 2 Li(s) + 1 I2(s) →2 LiI(s)
Lithium-iodine batteries are shaped like very small coins, as shown in the figure below:

The other type of cell or battery is the lithium ion. It takes this name precisely because its operation is based on the movement of lithium ions (Li+). It is currently widely used in cell phone batteries and its potential varies between 3.0 and 3.5 V.

The anode and cathode are formed by atoms arranged in planes like sheets with spaces where lithium ions are inserted. O anode is formed by graphite with copper metal and the ions are intercalated in the planes of hexagonal carbon structures, forming the following substance: readyÇ6.already the cathode is formed by lithium ions intercalated in an oxide with a lamellar structure (readxCoO2).
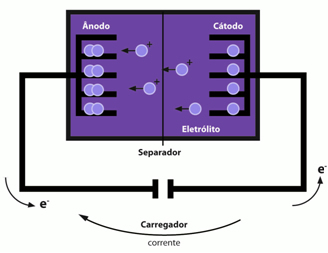
Thus, we have that lithium ions leave the anode and migrate through a non-aqueous solvent to the cathode.
Anode Half Reaction: LiyÇ6(s) → y Li + Ç6+y-
Cathode Semi-Reaction: LixCoO2(s) + y read+(s) + y and- → I readx+yCoO2(s)
Global Reaction: LiyÇ6(s) + readxCoO2 → Ç6(s) +readx+yCoO2(s)
These batteries are rechargeable, simply using an external electrical current that causes the migration of lithium ions in the opposite direction, that is, from the oxide to the graphite.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/pilhas-baterias-litio.htm

