Amines are nitrogenous organic compounds that are derived from the substance ammonia (NH3) by replacing one or more hydrogens with organic radicals. According to the amount of substituted hydrogens, the amines can be classified into:
Primary amine: formed by replacing a hydrogen in ammonia with an organic radical;
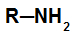
General structure of a primary amine
Secondary amine: formed by the replacement of two ammonia hydrogens by two organic radicals.

General structure of a secondary amine
Tertiary amine: formed by replacing the three hydrogens of ammonia with three organic radicals.

General structure of a tertiary amine
Physical Characteristics of Amines
They are polar compounds;
Primary and secondary amines are able to perform hydrogen bonds;
Tertiary amines perform interaction permanent dipole;
Amines with up to five carbons are soluble in water and ethanol. Amines with six or more carbons are practically insoluble in water, but soluble in organic solvents;
Amines that have an aromatic structure are denser than water;
Amines with one to three carbons are gaseous at room temperature. Those with four to twelve carbons are liquid at room temperature;
In general, amines have higher melting and boiling points only in relation to any non-polar organic compound.
Chemical Characteristics of Amines
They are considered organic bases, according to the Bronsted-Lowry theory;
Aromatic amines have a lower basic character due to the phenomenon of resonance;
The greater the basic character of the amine, the more likely it is to react with a certain substance;
In the presence of an acid, amines carry out neutralization reaction, since they have a basic character.
rule of amine nomenclature
To name an amine, simply follow the rule established by the International Union of Pure and Applied Chemistry (IUPAC), described below:
Name of the radical or radicals + amine
NOTE: If the amine has different radicals, we must follow alphabetical order.
Follow some examples:
Example 1: primary amine

In the primary amine in this example, we have the presence of a propyl radical, so its name is propylamine.
Example 2: secondary amine

In the secondary amine in this example, we have the presence of the methyl radical (to the left of the nitrogen) and the ethyl radical (to the right of the nitrogen). Its name is, following alphabetical order, ethyl-methylamine.
Example 3: tertiary amine
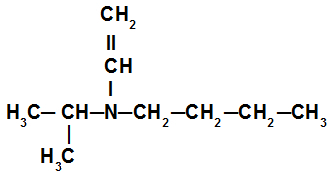
In the secondary amine in this example, we have the isopropyl radical (to the left of nitrogen), the butyl radical (to the right of the nitrogen), and the vinyl radical (below the nitrogen). Its name is therefore butyl-isopropyl-vinylamine.
Amine Applications
Widely used in the production of various organic compounds;
Used in the manufacture of soaps;
Used in the rubber vulcanization process;
Used in the manufacture of dyes.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-sao-aminas.htm