Chemistry is the science that studies matter, its structure, formation and the transformations it undergoes, taking into account the energy involved in the entire process.
Chemistry is part of the Natural Sciences and focuses on observing phenomena, creating theories to explain them and models that represent them.
Check the text that the All Matter prepared for you to know more about this science and its importance for humanity.
What does Chemistry study?
THE matter it is the object of study of Chemistry, which in turn establishes the relationships between its constitution, properties and transformations.
It is worth remembering that matter is everything that has mass and occupies a place in space. A material is composed of atoms, which group together and form the different existing chemical substances.
All efforts to develop this science allowed the human being to know the matter and the means to transform it, so that the knowledge could be used for our benefit.
Importance of Chemistry
Chemistry is present everywhere, but sometimes it is difficult to recognize it. From the cultivation of wheat to bread at our table, at various stages chemical knowledge has allowed us to improve manufacturing methods and quality of life.
Another example is that we receive materials such as food, water and air into our bodies. Inside our organism there are several chemical transformations so that we can take advantage of nutrients, produce energy and use oxygen to keep us alive.
Through Chemistry it is possible to study natural substances and make use of them. But also, materials can be produced in the laboratory.
See the example of sneakers, a product that contains materials, of natural or synthetic origin, that were transformed by man to create a consumer good for society.

Simply put, we can say that sneakers are made of rubber, fabric and metal.
- Metals are ores extracted from nature.
- Rubber can be natural and produced from the sap of rubber trees. Synthetic rubber, on the other hand, is made from petroleum.
- Natural fabric comes from cotton, while an example of synthetic fabric is nylon.
Chemistry has already been seen as a villain, due to the pollution coming from the means used to urgently supply the market and, for a long time, neglecting the environmental issue. Toxic, non-degradable products and industrial waste dumping are one of the many problems linked to chemicals.
However, this conception is changing. Green Chemistry encourages cleaner production, environmental preservation and industrial processes with less waste generated. Recycling, biofuels and a reduction in greenhouse gas emissions are some of the measures that we can already observe in our daily lives.
What is Chemistry for?
Chemical knowledge generates applications and technologies allow new products to be created. Chemistry is present in food, medicine, clothing, buildings, and so on.
Check out an example of where chemical knowledge was employed.
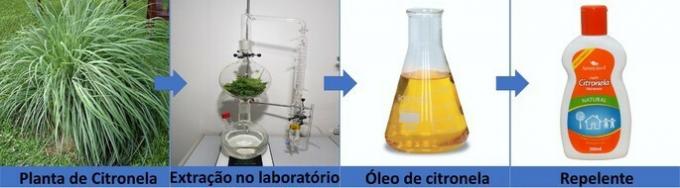
The active ingredient in the repellent is extracted from a plant called citronella. Through laboratory equipment and extraction techniques, chemists were able to isolate the oil from citronella and, together with other chemical substances, transformed into a product that prevents the sting of mosquitoes.
For this, it was necessary to study the composition of the substance, how it works and what its risks are. It's all part of chemistry: researching, investigating, experimenting and creating products that improve people's lives.
Although it is common to associate chemical knowledge with wars, because of the creation of chemical and atomic bomb, Chemistry has had important contributions throughout history. Some of them were:
- Alternative energy sources: discovery of the radioactivity of chemical elements and creation of nuclear energy to generate electrical energy.
- Industrialized foods: discovery of substances that preserve food and, thus, increased the shelf life of commercialized foods.
- Medicines: discovery of chemical substances capable of controlling and fighting diseases.
Main areas of Chemistry
| General chemistry | Concepts and terms that are the basis for understanding the other areas. |
|---|---|
 |
Example:
|
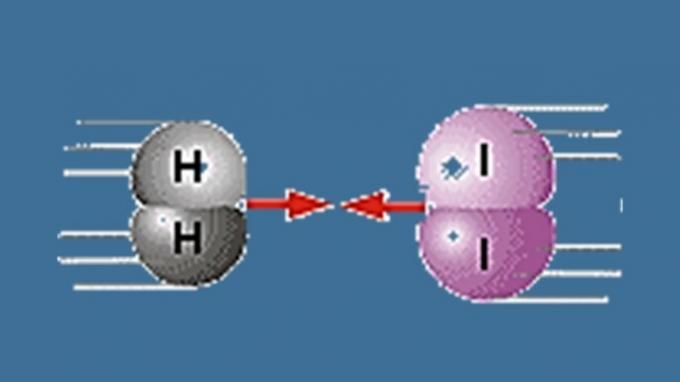
| Physicochemical | Studies the energy and dynamics of chemical transformations. |
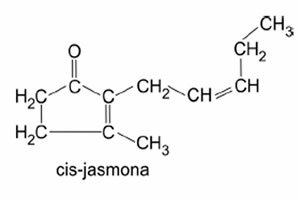
 |
Example:
|
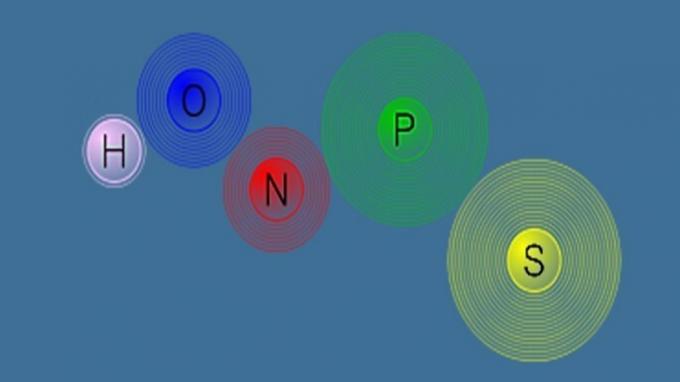
| Inorganic chemistry | Study the compounds formed by chemical elements. |
 |
Example:
|
| Organic chemistry | Study the compounds formed by carbon. |
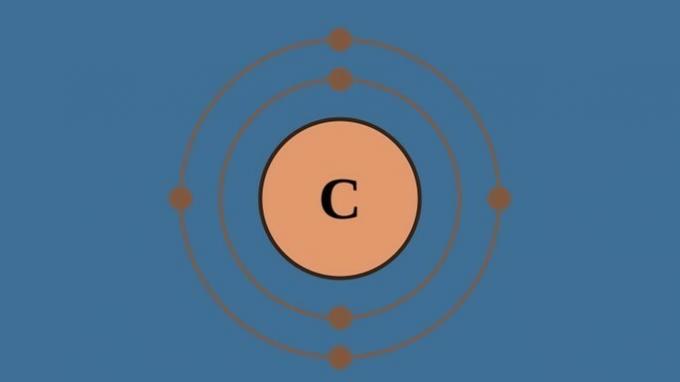 |
Example:
|
| Nuclear Chemistry | Study the reactions in the nuclei of atoms. |
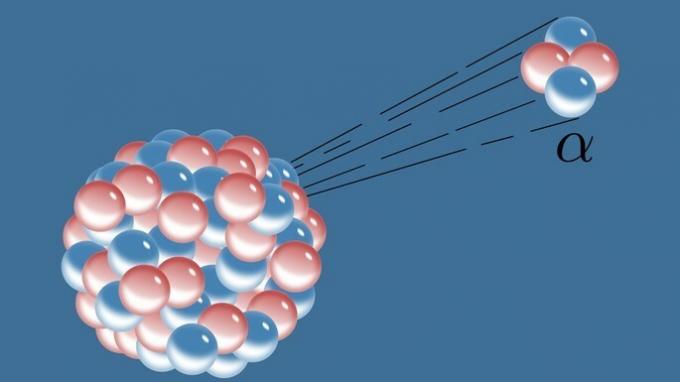 |
Example:
|
| Environmental Chemistry | Study the chemical processes in the environment. |
 |
Example:
|
Do you want to know specific areas of Chemistry? So check out these texts:
- Biochemistry
- Organic chemistry
- Inorganic chemistry
History of Chemistry
The precursor of Chemistry is the Alchemy, a widespread practice in the Middle Ages, which involved science, art and magic. For some, in the Arabic language, the term “Alchemy” (Al-Khemy) means “chemistry”.

Alchemy's goal was to create the Philosopher's Stone, capable of transforming common metals into gold, and produce the Elixir of Immortality, which would cure all ills and guarantee long life.
In this search, many chemical substances were created and laboratory equipment was made to carry out experiments.
The knowledge acquired by alchemists was important to support modern Chemistry, which emerged in the 18th century.
Gradually, scholars were abandoning alchemist theories and adopted experimental methods to explain the observed phenomena.
Lavoisier he is considered the father of modern chemistry for his significant contribution to the emergence of this science and consolidation of the scientific method as a new way of studying chemical processes.
To acquire more knowledge, be sure to read these texts.:
- scientific method
- Materials used in the Chemistry laboratory
- laboratory glassware