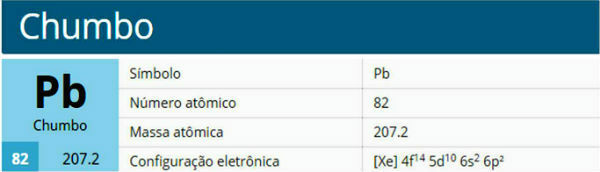
Lead is a chemical element with atomic number 82, atomic mass 207.2 and belonging to group 14 of the periodic table.
It is characterized by being a heavy, toxic and malleable metal.
At room temperature, it is found in a solid state, with a bluish-white color and which in contact with air becomes grayish. It is also a poor conductor of electricity and very resistant to corrosion.
Lead in its elemental form is rarely found in nature. Thus, it is more common to find it in minerals such as galena, anglesite and cerusite.
applications
Lead has several types of uses, being found in numerous products. It is a metal used since ancient times by man.
We can list the presence and usefulness of lead in various industries and products:
- Various equipment and utensils in industries and construction;
- Ammunition;
- Cosmetics and pigments, especially lipsticks and hair dyes. Due to its toxicity, some countries have banned its presence in cosmetics;
- Metal alloys;
- Fuel additive. In 1992, Brazil banned the use of lead in gasoline because when released into the air it causes environmental contamination.
- Radiation shielding blankets;
- Production of welds.
Also read about:
- Chemical elements
- Periodic table
Intoxication
Lead is a harmful element for human health and contact with the metal occurs orally, inhaled or through the skin.
Children and pregnant women are more susceptible to lead poisoning.
Some cases of poisoning can occur through domestic utensils coated with copper-based ceramic enamels. When it comes into contact with acidic substances, lead can leach into food.
It is important to emphasize that lead does not decompose over time nor is it degraded by the effect of heat. In addition, it has the ability to accumulate in the body, especially in the kidneys, liver, brain and bones.
Lead can cause vomiting, abdominal pain, seizures, encephalopathy, muscle weakness, and kidney, liver, and brain damage.
The level of lead in the body can be checked with a blood test and in some cases x-rays. The intoxicated person must receive medical assistance.


