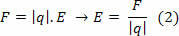The thermal capacity (C), also called heat capacity, is a quantity that corresponds to the amount of heat present in a body in relation to the temperature variation suffered by it.
Formula
To calculate the thermal capacity, the following formula is used:
C = Q/Δθ or C = m. ç
Where,
Ç: thermal capacity (cal/°C or J/K)
Q: amount of heat (lime or J)
Δθ: temperature variation (°C or K)
m: mass (g or kg)
ç: specific heat (cal/g°C or J/Kg. K)
Example
If a body has a heat capacity of 20 cal/°C, this means that when it receives or gives away 20 calories, its temperature will increase or decrease by 1°C.
Thermal Capacity and Specific Heat
O specific heat (c), also called thermal mass capacity, is a physical quantity that is related to the amount of heat received by a body and its thermal variation.
Therefore, it determines the amount of heat needed to increase the temperature by 1 °C of 1g of the element.
Unlike specific heat, which depends only on the substance, heat capacity is directly dependent on the substance and the mass of the body.
In other words, the thermal capacity (C) is a physical quantity characteristic of the body, that is, it intervenes in its mass. On the other hand, specific heat (c) is a physical quantity characteristic of substance.
To calculate the specific heat of substances, the following formula is used:
c = Q/m. Δθ or c = C/m
Where,
ç: specific heat (cal/g°C or J/Kg. K)
Q: amount of heat (lime or J)
m: mass (g or kg)
Δθ: temperature variation (°C or K)
Ç: thermal capacity (cal/°C or J/K)
Read too:
- Calorimetry
- Heat and Temperature
- Sensitive heat
- latent heat
Entrance Exam Exercises with Feedback
1. (PUCCAMP) A copper bar of mass 200 g is removed from the interior of an oven, where it was in equilibrium heat, and placed inside a container with a thermal capacity of 46 cal/°C containing 200 g of water at 20°C. The final equilibrium temperature is 25°C. The oven temperature, in °C, is approximately equal to: Given: CCu = 0.03 cal/g°C
a) 140
b) 180
c) 230
d) 280
e) 300
Alternative c
2. (UFSE) The table below shows the mass m of five metal objects, with their respective sensitive specific heats c.
| Metal | c (cal/g°C) | m (g) |
|---|---|---|
| Aluminum | 0,217 | 100 |
| Iron | 0,113 | 200 |
| Copper | 0,093 | 300 |
| Silver | 0,056 | 400 |
| Lead | 0,031 | 500 |
The object that has the greatest thermal capacity is:
a) aluminum
b) iron
c) lead
d) silver
e) copper
Alternative and
3. (Mackenzie) A thermal source delivers 55 cal/s at constant power. A body of mass 100 g fully absorbs the energy from the source and has a temperature that varies as a function of time, as shown in the graph below.

The thermal capacity of this body and the specific heat of the substance of which it is constituted are, respectively, equal to:
a) 2.2 cal/°C and 0.022 cal/g°C.
b) 2.2 cal/°C and 0.22 cal/g°C.
c) 2.2 cal/°C and 2.2 cal/g °C.
d) 22 cal/°C and 0.22 cal/g°C.
e) 22 cal/°C and 0.022 cal/g°C.
Alternative