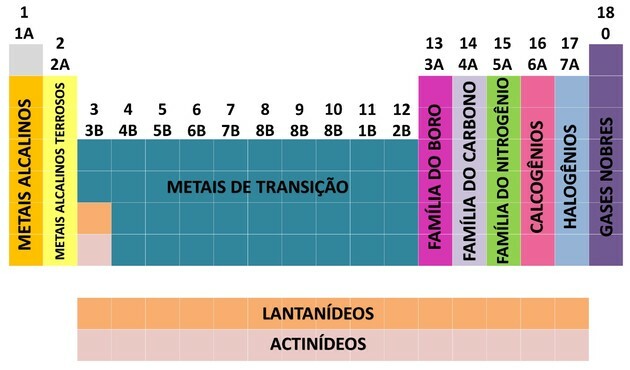
One of the ways chemical elements are organized is through families, which correspond to the vertical sequences of the periodic table.
At 18 columns of the table group the elements according to similarities in chemical properties.
Organizing chemical elements into families was a practical way of structuring the various information found and presenting them in a simple way.
To facilitate the location of a chemical element, families were designated in numbers from 1 to 18 as follows:
Through the contribution of many scientists and various attempts to arrange the data, the periodic table evolved, establishing an order to arrange the elements.
Nomenclature of families
- The families in the table were divided into A (representative) and B (transition), being identified by letters and numbers.
- You representative elements families 0, 1A, 2A, 3A, 4A, 5A, 6A and 7A correspond.
- You transition elements families 1B, 2B, 3B, 4B, 5B, 6B, 7B and 8B correspond.
- By determination of the International Union of Pure and Applied Chemistry (IUPAC), the families were identified in groups from 1 to 18.
Periodic Table and Electronic Distribution
The similarities between the elements of the same family occur because the number of valence electrons of the atom in the ground state is the same for the members of a certain group.
For example:
| Group 1 | Eletronic distribution |
|---|---|
| 3read | 2-1 |
| 11At | 2-8-1 |
| 19K | 2-8-8-1 |
| 37Rb | 2-8-18-8-1 |
| 55Cs | 2-8-18-18-8-1 |
| 87Fr | 2-8-18-32-18-8-1 |
Group 1 atoms have their electrons distributed over more than one energy level, but all have a valence electron.
With that, we observe that making the eletronic distribution of the atom in the ground state, we find its position on the periodic table.
representative elements
Representative elements exhibit relatively less complex chemical behavior than transition elements and form most of the substances around us.
Some of the representative element families are given special names, as shown below:
Group |
Family | specific name | Name origin | Elements | electronic configuration |
|---|---|---|---|---|---|
| 1 | 1A | alkali metals | from latin alkali, which means “gray of plants”. | Li, Na, K, Rb, Cs and Fr | us1 |
| 2 | 2A | alkaline earth metals | The term "earthly" refers to "existing on earth". | Be, Mg, Ca, Sr, Ba and Ra | us2 |
| 13 | 3A | boron family | Name of the first element of the family. | B, Al, Ga, In, Tl and Nh. | us2np1 |
| 14 | 4A | carbon family | Name of the first element of the family. | C, Si, Ge, Sn, Pb and Fl. | us2np2 |
| 15 | 5A | Nitrogen family | Name of the first element of the family. | N, P, As, Sb, Bi and Mc. | us2np3 |
| 16 | 6A | Chalcogens | from the greek khalks, as they are elements found in copper ores. | O, S, Se, Te, Po and Lv. | us2np4 |
| 17 | 7A | Halogens | Greek expression meaning salt-formers. | F, Cl, Br, I, At and Ts. | us2np5 |
| 18 | 0 | Noble Gases | It was considered not to react with other substances. | He, Ne, Ar, Kr, Xe, Re and Og. | 1s2 (He) or us2np6 |
Through the table, we can see that:
- The elements presented above are classified as representative because they have the most energetic electron in an s or p sublevel.
- Electrons are distributed over energy levels and n represents the outermost level of the atom in the ground state.
- The representative elements, according to the recommendation of the IUPAC, belong to the groups or families 1,2,13,14,15,16,17 and 18.
O hydrogen it is classified apart from the other elements. Even with 1s electronic configuration1, he is not part of group 1 for presenting a singular behavior.
transition elements
The transition elements correspond to groups 3 to 12. They receive this name because they have intermediate characteristics between groups 1 and 2 and the representative non-metallic elements.
You transition metals are defined by IUPAC as:
A transition element has an incomplete d sublevel or may form cations with an incomplete d sublevel.
When the most energetic electron of the ground state atom is in an incomplete d sublevel, it is characterized as an outer transition.
Lanthanides and actinides are internal transition elements as they have at least one incomplete f sublevel.
When elements have electrons that fill the d or f orbitals, they exhibit similar properties and can be classified into d or f orbital elements. outer or inner transition.
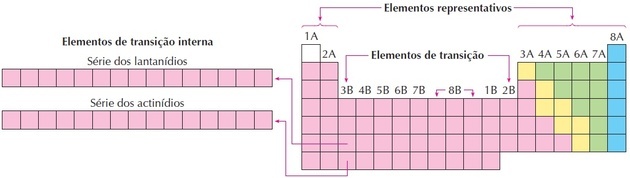
As we can see in the image, family 8B corresponds to 3 columns, they are groups 8, 9 and 10, which were grouped like this because they have similar characteristics.
Main characteristics of families
The table below shows the main properties of the groups in the periodic table:
| Group | Features | compound substances more common |
occurrences |
|---|---|---|---|
| 1 | Solid and shiny in ambient conditions. Very reactive, soft and good conductors of electricity. | Salts, hydroxides and oxides |
React with halogens and form salts. |
| 2 | Less reactive and harder than group 1. Silver solids with good conductivity. | Salts, hydroxides and oxides |
Forming salts and oxides. |
| 3 to 12 | They form complexes. They are metallic solids, hard and brittle, with the exception of mercury, which is a liquid. | Salts, oxides and complexes. Ex: AgNO3, TiO and [Cr(OH)3(H2O)3] |
In minerals in the form of oxides. |
| 13 | Solids in ambient conditions, silver, except for boron. | Oxides Ex: B2O3 |
In minerals in the form of oxides. |
| 14 | Solid in ambient conditions. | Atoms of C and Si can arrange themselves in chains and produce a huge variety of substances. | In living organisms and in the form of silicates or oxides. |
| 15 | Solids, except nitrogen, which is gaseous under ambient conditions. | Oxides and acids Ex: NO2 and H3DUST4 |
Atmosphere, living organisms and minerals. |
| 16 | Solids, except oxygen, which is gaseous under ambient conditions. | Sulfides and oxides Ex: ZnS and SiO2 |
Atmosphere, living organisms and minerals. |
| 17 | They form diatomic molecules and are very reactive. They are bad conductors of electricity and heat. They are aggressive to living beings and the environment. | Acids and Salts. Ex: HCl and KBr |
Are present in substances organic and mineral. |
| 18 | They are very stable and are found in the form of gases. | They hardly form compound substances. | Gases in the atmosphere. |
Chemical and physical properties distinguish one family from another. As we have seen, chemical properties are related to electrons of valence, and through them, an atom interacts with another, being responsible for the chemical behavior and chemical bonds formed.
The physical properties of elements in the same group can vary according to atomic number and mass.
Exercises
Now that you know a little more about the Periodic Table families, test your knowledge and see what you've learned.
1) Consider the following extract from the Periodic Table.
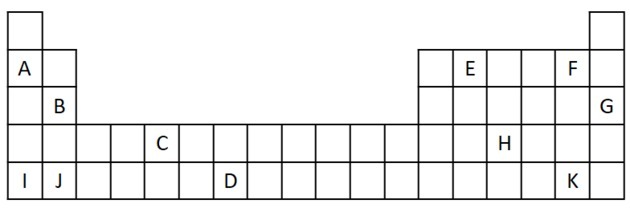
a) Name two elements that have two valence electrons.
b) Indicate an element that reacts violently with water, producing a metal hydroxide.
c) Indicate a non-reactive element.
d) Indicate two elements that combine with alkali metals to form salts.
Reply:
a) B and J
Two valence electrons correspond to group 2, which has an ns electron configuration2 and are represented in the exercise by B and J.
b) A, B, I or J.
A and I represent elements of family 1; B and J are from family 2. As we saw in the properties table, group 1 and 2 elements are very reactive and can form hydroxides, as in the examples given: KOH and Mg (OH)2.
c) G
Noble gases are very stable and therefore not very reactive. The letter G is inserted in the table as an element of this family.
d) F and K
Halogens react with alkali metals to form salts. The most common example of this is table salt, NaCl.
2) The following figure shows three chemical elements, from left to right, lithium, sodium and potassium.

Select the option that correctly completes each of the following statements.
1.1) "We can say that lithium, sodium and potassium...
(A) … belong to the same period.”
(B) …has the same atomic number.”
(C) … belong to the same group.”
(D) …has the same mass number.”
1.2) "The elements lithium, sodium and potassium...
(A) …has very similar chemical properties.”
(B) …has very different chemical properties.”
(C) … are non-metals.”
(D) … react with water to form acidic solutions.”
Answers:
1.1) (C) belong to the same group.
1.2) (A) has very similar chemical properties.
We can obtain this information by consulting the periodic table or by making the electronic distribution of the elements lithium, sodium and potassium. We will see that the three have an electron in the valence shell and because they are part of the same group, the chemical properties are very similar.
3) Consider the following table, where the atomic numbers and electronic distributions of some chemical elements are represented.
| Element | chemical symbol | atomic number | Eletronic distribution |
|---|---|---|---|
| Lithium | read | 3 | 2,1 |
| Beryllium | be | 4 | 2,2 |
| Fluorine | F | 9 | 2,7 |
| neon | Huh | 10 | 2,8 |
| chlorine | Cl | 17 | 2,8,7 |
| argon | Air | 18 | 2,8,8 |
| Potassium | K | 19 | 2,8,8,1 |
| Calcium | Here | 20 | 2,8,8,2 |
Indicate the group of each element.
Reply:
Lithium and potassium: group 1. (They have an electron in the valence shell).
Beryllium and calcium: group 2. (They have two electrons in the valence shell).
Fluorine and chlorine: group 17. (They have seven electrons in the valence shell).
Neon and argon: group 18. (They have eight electrons in the valence shell).
Check entrance exam questions with a commented resolution in Exercises on the Periodic Table and unpublished questions on the subject in Exercises on Organizing the Periodic Table.

