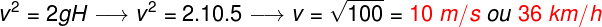Zero Law of Thermodynamics is the one that deals with the conditions for two bodies (A and B) to obtain the thermal balance with a third body (C).
A thermometer (body A) in contact with a glass of water (body B) and, on the other hand, a thermometer in contact with a cup containing water and ice (body C) obtain the same temperature.
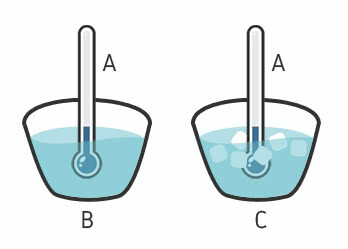
If A is in thermal equilibrium with B and if A is in thermal equilibrium with C, then B is in thermal equilibrium with C. This happens even though B and C are not in contact.
That's what happens when we put two bodies with different temperatures in contact. Heat is the energy transferred from the body with the highest temperature to the body with the lowest temperature.
Let's imagine a hot cup of coffee. You are in a hurry to take it and then you need to cool down so you don't burn yourself. So, add milk to coffee.
Coffee temperature (T1) is higher than the temperature of the milk (T2), ie T1 > T2.
But now we have coffee with milk, whose temperature is due to the contact of T
1 and T2, after some time, results in T3, which means that it reached the thermal balance. So we have to T1 > T3 > T2.The temperature is influenced by the type of material it is made from. In other words, the temperature depends on the thermal conductivity, larger or smaller on different materials.
Thermometers were invented to correctly measure temperature, after all, sensory perception was not effective.
There are three temperature scales: Celsius (°C), Kelvin (K) and Fahrenheit (°F). Learn more at Thermometric Scales.
It should be noted that the Zero Law of Thermodynamics was postulated after the first laws of thermodynamics, the First Law of Thermodynamics and the Second Law of Thermodynamics.
It was because it was necessary for the understanding of these laws that it received a name that preceded them.
Read too: Thermodynamics and Physics Formulas.
Solved Exercises
1. (UNICAMP) An efficient thermal insulation is a constant challenge to be overcome so that man can live in extreme temperature conditions.
For this, a complete understanding of the heat exchange mechanisms is essential. In each of the situations described below, you must recognize the heat exchange process involved.
I. The shelves of a domestic refrigerator are hollow grids, to facilitate the flow of thermal energy to the freezer for […]
II. The only heat exchange process that can take place in a vacuum is […].
II. In a thermos, a vacuum is maintained between the double walls of glass to prevent heat from exiting or entering through [….].
In order, the heat exchange processes used to fill the gaps correctly are:
a) conduction, convection and radiation.
b) conduction, radiation and convection.
c) convection, conduction and radiation.
d) convection, radiation and conduction.
Alternative d: convection, radiation and conduction.
2. (VUNESP-UNESP) Two identical glass cups, in thermal equilibrium with room temperature, were stored, one inside the other, as shown in the figure.
One person, trying to disengage them, was unsuccessful. To separate them, he decided to put his knowledge of thermal physics into practice.

According to thermal physics, the only procedure capable of separating them is:
a) immerse cup B in thermally balanced water with ice cubes and fill cup A with water at room temperature.
b) put hot water (greater than room temperature) in cup A.
c) immerse cup B in ice water (below room temperature) and leave cup A without liquid.
d) fill cup A with hot water (above room temperature) and immerse cup B in ice water (below room temperature).
e) fill cup A with cold water (below room temperature) and immerse cup B in hot water (higher than room temperature).
Alternative e: fill cup A with ice water (below room temperature) and immerse cup B in hot water (higher than room temperature).
See too: Exercises on Thermodynamics