Boiling is the change from a liquid to a gaseous state. It happens when a portion of liquid, subjected to a given pressure, receives heat and reaches a certain temperature.
The amount of heat that the body must receive to transform itself completely into vapor depends on the substance that constitutes it.
A substance in the liquid state does not have a defined form, assuming the shape of the container that contains it.
Being practically incomprehensible, it presents a cohesive force between the particles that constitute it.
To pass to the gaseous state, the substance must receive heat. This increase in energy will make the molecules vibrate with greater intensity, increasing the distance between them.
In this way, the cohesive force becomes practically non-existent. The body in this state has no definite shape or volume.
Geysers are examples of boiling that occurs with groundwater located in volcanic regions. The magma heats the water and when it reaches a certain temperature it starts to change state.
The steam occupies a greater volume, increasing the pressure in the underground cavity. As a result, a mixture of vapor and liquid is expelled to the surface through small cracks.

Boiling Characteristics
A liquid boils in the following pattern:
- Keeping the pressure constant, the temperature throughout the boiling process will remain constant.
- The amount of heat per unit of mass required for a liquid to completely transform into vapor is called the latent heat of vaporization. Its value depends on the substance that makes up the liquid.
- The temperature at which each substance boils is well determined, and is called the boiling point.
Tip: When we're cooking food, it's a good idea to turn the heat on low when the water starts to boil. As the temperature remains constant throughout the boiling process, the cooking time will be the same with high heat or low heat. In this way, we save gas and the environment is grateful.
Amount of Latent Heat
The amount of heat that a liquid must receive to transform into vapor depends on the value of the latent heat of vaporization and its mass.
Below we present the value of the latent heat of vaporization of some substances:

Formula
To calculate the amount of heat needed for a liquid to change state, we use the following formula:
Where,
Qv: amount of heat (lime)
m: mass (g)
Lv: latent heat of vaporization (cal/g)
Example:
How much heat is needed for 100 g of Ethanol to boil and turn completely into steam?
Qv = 100. 204 = 204 000 cal
Boiling Temperature
The temperature at which a body undergoes boiling depends on the substance that composes it and the pressure it is subjected to.
The boiling point of substances is determined in the laboratory. For example, the boiling point of water, subjected to 1 atmosphere, is 100°C. Iron is 2800 ºC, while hydrogen is - 252.8 ºC.
To know the phase change temperature of other substances, read also boiling point.
The less pressure a body is subjected to, the lower its boiling point. This means that in cities with high altitudes, it takes much longer to cook food.
To cook food faster, we use pressure cookers. This type of cooker uses a sealing system that makes the pressure inside it to be greater than atmospheric pressure.
The higher pressure makes the boiling point higher as well. In the case of water, it will boil at a temperature that can reach 120 ºC, reducing cooking time.
phase changes
The change from a liquid to a gaseous state is generically called vaporization, as it encompasses, in addition to boiling, two other processes: evaporation and heating.
Evaporation occurs gradually, not needing to reach a specific temperature to occur. On the other hand, heating occurs when we place the liquid on a surface that is at a temperature above its boiling point.
There are still other processes of change of state. Are they:
- Fusion
- Solidification
- Liquefaction or Condensation
- Sublimation
In the diagram below we represent the three physical states of matter and the respective changes of state:
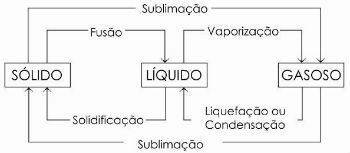
To learn more, read too Physical States of Water.
Exercises
Enem - 1999
The text should be used for the following two questions.
The pressure cooker allows food to be cooked in water much faster than conventional cookers. Its lid has a rubber seal that does not let steam escape, except through a central hole on which a weight that controls the pressure rests. When in use, a high pressure develops inside. For its safe operation, it is necessary to observe the cleanliness of the central hole and the existence of a safety valve, normally located in the cover.
The pressure cooker schematic and a water phase diagram are presented below.
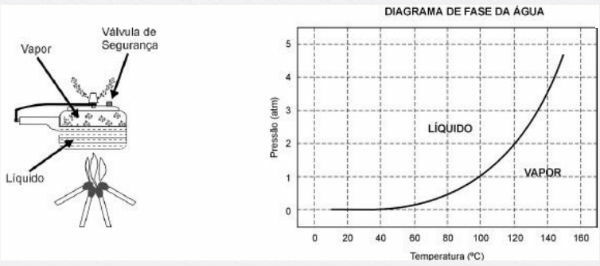
1) The advantage of using a pressure cooker is the speed of cooking food and this is due to
a) the pressure inside it, which is equal to the pressure outside.
b) the temperature of its interior, which is above the boiling temperature of the water in the place.
c) the amount of additional heat that is transferred to the pan.
d) the amount of steam being released by the valve.
e) the thickness of its wall, which is greater than that of common pans.
Alternative b: at its interior temperature, which is above the boiling temperature of the water in the place.
2) If, for economy, we lower the heat under a pressure cooker as soon as the steam comes out through the valve, in order simply to keep the boiling time, the cooking time
a) it will be bigger because the pan “cools”.
b) will be smaller, as it reduces water loss.
c) will be greater as the pressure decreases.
d) will be greater as evaporation decreases.
e) will not change as the temperature does not change.
Alternative e: will not be changed as the temperature does not vary.