Changes in physical state depend on some factors related to temperature, pressure, and the amount of energy involved in the process.
In nature, there are three physical states of matter: solid, liquid and gas. And physical state changes represent the five processes of change from one state to another.
The changes are: condensation or liquefaction, solidification, fusion, vaporization and sublimation. Each type of change has some specificities and are related to the qualities of the matter.
Condensation
Condensation represents the passing of the state gaseous to liquid.
This occurs due to the cooling of a gas, which tends to condense and become a liquid.
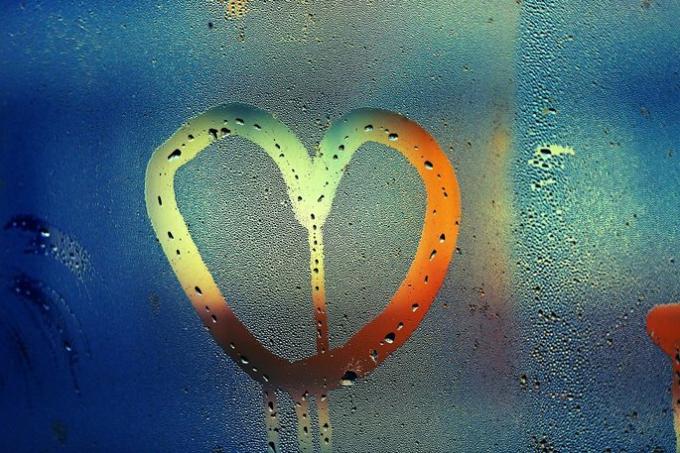
Solidification
Solidification is the passage of liquid to solid state.
A matter in a liquid state, if cooled tends to become solid. In the case of water, solidification occurs at 0 °C

Fusion
The fusion is the passage of the solid state to liquid.
The molecules of each substance need a certain amount of energy to move. When there is less energy, they tend to move less and the material tends to be solid.
When receiving energy from a heat source (heating), they become more agitated and can change state.

Vaporization
Vaporization is the passage of liquid to gaseous state. This can happen in two ways:
- Boiling: rapid heating.
- Evaporation: slow heating.
From 1 °C to 100 °C, it is liquid.

Sublimation
Sublimation is the passage of solid state to gaseous It's from gaseous state to solid (ressublimation).
This type of change occurs depending on certain pressure and temperature conditions. Each element has its Phases diagram, where its fusion, vaporization and sublimation curves are located.

Physical states of water
Water is easily found in its three physical states: solid, liquid and gas.
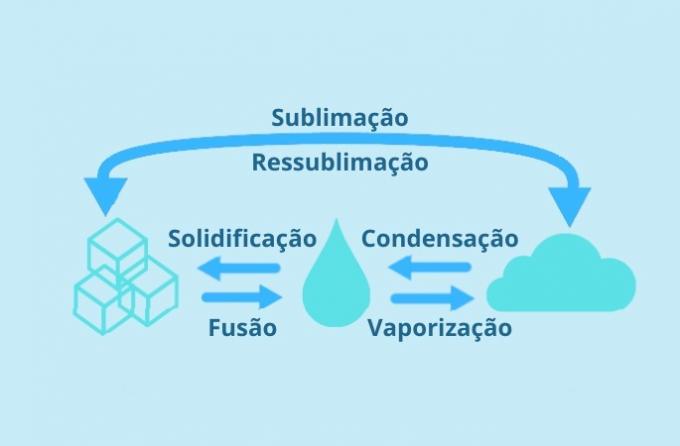
Each physical state of water is possible according to variations in temperature and pressure.
At normal pressure (1atm), water melts at 0 °C and boils at 100 °C.
Molecules of water at -1 °C are in a solid state and at 0 °C the change (melting point) from ice at 0 °C to water at 0 °C occurs.
When it reaches a temperature of 100 °C, it performs a new change of state (vaporization), going from a liquid to a gaseous state.
As can be seen from its phase diagram:
To learn more about this subject, read also:
- Physical States of Water
- Physical States of Matter
- Physical and chemical transformations
- Physical and chemical phenomena