defines itself as hybridization the union of incomplete atomic orbitals. An orbital is classified as incomplete when it has only one electron inside it instead of two. See a representation of an incomplete orbital and a complete orbital:

In image A, we have a complete orbital; in image B, an incomplete orbital.
Hybridization is a natural phenomenon that occurs with some chemical elements, such as Phosphorus, Sulfur, Carbon, etc. The hybridization of carbon allows the atoms of this element to be capable of making four chemical bonds, that is, carbon only makes four bonds after undergoing the phenomenon of hybridization.
But why does carbon hybridize and make four bonds? To understand this phenomenon, we have to know the electronic distribution of this element:
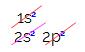
In the electronic distribution of carbon, we see that the 1s sublevel is complete (with two electrons), the 2s is complete (with two electrons) and the 2p sublevel is incomplete (the p sublevel supports six electrons, but there is only two). Distributing the 2p electrons in a representative way, we have to:
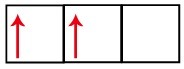
The p sublevel has three orbitals – in the case of carbon, two of them are incomplete and one is empty. For this reason, this element should only perform two links, as the number of incomplete orbitals always determines the number of links.
However, when receiving energy from the external environment, the electrons present in the carbon are excited. Thus, one of the electrons present in sublevel 2s moves to the orbital of sublevel p that was empty:
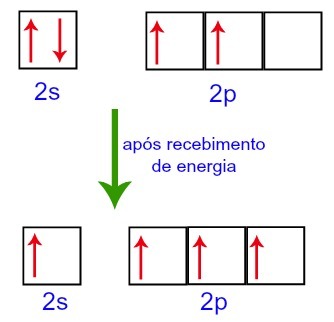
Carbon Orbitals before and after receiving energy from the external environment
Thus, carbon is left with four incomplete orbitals in its second level. Then, the orbital of the 2s sublevel joins the three p orbitals, which configures the phenomenon of hybridization.
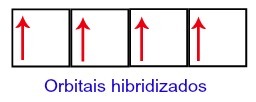
Carbon orbitals after hybridization
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/hibridizacao-carbono.htm


