Niobium (Nb) is the chemical element of atomic number 41 belonging to group 5 of the periodic table.
It is a naturally available transition metal in solid state, which was discovered in 1801 by British chemist Charles Hatchett.
Minerals containing niobium are rare in the world, but abundant in Brazil, the country with the largest reserves of this metal.
Due to its properties, high conductivity and corrosion resistance, this element has many applications ranging from steel production to rocket manufacturing.
Next, we'll introduce this chemical element and the characteristics that make it so important.
What is niobium?
Niobium is a refractory metal, that is, it is very resistant to heat and wear.
The metals in this class are: niobium, tungsten, molybdenum, tantalum and rhenium, with niobium being the lightest of all.
Niobium occurs in nature in minerals, usually linked to other elements, mainly tantalum, as the two have very similar physicochemical properties.
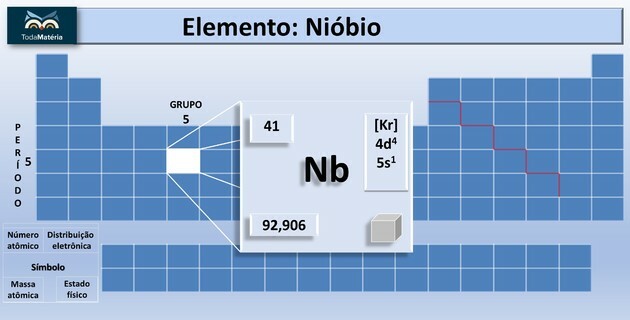
This chemical element is classified as a transition metal on the periodic table. It is bright, of low hardness, with low resistance to the passage of electric current and resistant to corrosion.
Physical Properties of Niobium
| physical state | solid at room temperature |
|---|---|
| color and appearance | metallic gray |
| Density | 8.570 g/cm3 |
| Fusion point | 2468°C |
| Boiling point | 4742 °C |
| Crystalline structure | Body Centered Cubic - CCC |
thermal conductivity |
54.2 W m-1 K-1 |
Chemical Properties of Niobium
| Classification | transition metal |
|---|---|
| atomic number | 41 |
| Block | d |
| Group | 5 |
| Time course | 5 |
| atomic weight | 92,90638 u |
| atomic ray | 1,429 Å |
| common ions | Nb5+ and Nb3+ |
| electronegativity | 1.6 Pauling |
The main advantage of using this metal is that only a quantity, in grams, of this element can modify a ton of iron, making the metal lighter, resistant to corrosion and more efficient.
Where is Niobium found?
When compared to other substances present in nature, niobium has a low concentration, in the proportion of 24 parts per million.
This metal is found in the following countries: Brazil, Canada, Australia, Egypt, Democratic Republic of Congo, Greenland, Russia, Finland, Gabon and Tanzania.
Niobium in Brazil
In the 1950s, the largest deposit of pyrochlore ore, containing this metal, was discovered in Brazil by Brazilian geologist Djalma Guimarães.
The large amount of ores that contain niobium is located in Brazil, the world's largest producer, which holds more than 90% of the metal's reserves.
The explored reserves are located in the states of Minas Gerais, Amazonas, Goiás and Rondônia.
niobium ores
Niobium is found in nature always linked to other chemical elements. More than 90 mineral species containing niobium and tantalum in nature are already known.
In the table below, we can see some of the ores that contain niobium, the main characteristics and the niobium content available in each material.
| columbite tantalite | |
|---|---|
 | |
| Composition: | (Fe, Mn)(Nb, Ta)2O6 |
| Niobium content (maximum): | 76% of Nb2O5 |
| Features: |
|
| Pyrochlorite | |
|---|---|
 | |
| Composition: | (At2,Here)2(Nb, Ti)(O, F)7 |
| Niobium content (maximum): | 71% of Nb2O5 |
| Features: |
|
| Loparite | |
|---|---|
 | |
| Composition: | (C, Na, C)2(Ti, Nb)2O6 |
| Niobium content (maximum): | 20% of Nb2O5 |
| Features: |
|
niobium exploration
Niobium ores undergo transformations until the products that will be marketed are formed.
The process steps can be summarized as:
- Mining
- Niobium concentration
- Niobium refining
- Niobium products
Mining takes place where there are ore reserves, which are extracted using explosives and transported by belts to where the concentration stage takes place.
Concentration occurs with the breakdown of the ore, grinding makes the ore crystals become much finer and using the magnetic separation the iron fractions are removed from the ore.
In the refining of niobium, there is the removal of sulfur, water, phosphorus and lead.
One of the products containing niobium is the iron-niobium alloy, which is produced according to the following equation:
This process is called aluminothermia in which the ore concentrate is mixed in reactors with iron scrap or iron oxide.
Metal oxides react with aluminum under high temperatures, generating the product of interest.
The most commercialized niobium products are:
- Niobium concentrates: a base that contains 58% Nb2O5.
- Iron-niobium alloy: contains 65% niobium.
- High purity oxide: used in the production of special materials.
What is niobium for?
The characteristics of niobium make this element increasingly desirable and with countless applications.
Since its discovery in 1905, applications for niobium began to be investigated, when the German chemist Werner von Bolton produced the element in pure form.
The 50's represented a great search for niobium applications, as until then it was not produced on a large scale.
During this period, the cold war sparked interest in this metal to be used in aerospace components.
Below is a list of the ways in which niobium is used.
Metal alloys

The addition of niobium to an alloy increases its hardenability, that is, the ability to harden when exposed to heat and then cooled. Thus, the material containing niobium can be subjected to specific heat treatments.
The affinity of niobium with carbon and nitrogen favors the mechanical properties of the alloy, increasing, for example, mechanical strength and resistance to abrasive wear.
These effects are beneficial as they can extend an alloy's industrial applications.
Steel, for example, is a metallic alloy formed by iron and carbon. The addition of niobium to this alloy can have advantages for:
- Automotive industry: producing a car lighter and more resistant to collision.
- Construction: improves the weldability of steel and provides malleability.
- Transport pipeline industry: Allows constructions with thinner walls and larger diameters, without affecting safety.
super alloys

The superalloy is a metallic alloy with high resistance to high temperatures and mechanical strength. Alloys containing niobium make this material useful in the manufacture of aircraft turbines or energy production.
The advantage of operating at high temperatures makes super alloys compose high-performance jet engines.
superconducting magnets

The superconductivity of niobium causes the compounds of niobium-germanium, niobium-scandium and niobium-titanium to be used in:
- Scanner of MRI machines.
- Particle accelerators such as the Large Hadron Collider.
- Detection of electromagnetic radiation and study of cosmic radiation by materials containing niobium nitrite.
Oxides
Other applications for niobium are in the form of oxides, mainly Nb2O5. The main uses are:
- optical lenses
- Ceramic Capacitors
- pH sensors
- engine parts
- Jewelry
History and Discovery of Niobium
In 1734 some ores belonging to a personal collection of John Winthrop were taken from America to England and these items were part of the collection of the British Museum in London.
Upon joining the Royal Society, British chemist Charles Hatchett focused on investigating the composition of the ores available at the museum. This is how, in 1801, he isolated a chemical element, in the form of oxide, and named it columbium and the ore from which it was extracted columbite.
In 1802, Swedish chemist Anders Gustaf Ekeberg reported the discovery of a new chemical element and named it tantalum, in reference to the son of Zeus in Greek mythology.
In 1809, the English chemist and physicist William Hyde Wollaston analyzed these two elements and observed that they had very similar characteristics.
Due to this fact, from 1809 to 1846, columbium and tantalum were considered the same element.
Later, German mineralogist and chemist Heinrich Rose, investigating the columbite ore, noted that tantalum was also present.
Rose found the presence of another element, similar to tantalum and called it Niobius, in reference to Niobe, daughter of Tantalus, from Greek mythology.
In 1864, Swede Christian Bromstrand managed to isolate niobium from a sample of chloride heated in a hydrogen atmosphere.
In 1950, the Union of Pure and Applied Chemistry (IUPAC) approved niobium as the official name, rather than columbium, as they were the same chemical element.
Niobium Summary
Chemical element: Niobium | |||
|---|---|---|---|
| Symbol | Nb | Discoverer | Charles Hatchett |
| atomic number | 41 | atomic mass | 92,906 u |
| Group | 5 | Time course | 5 |
| Classification | transition metal | Eletronic distribution | [Kr]4d35s2 |
| Features |
|
||
| Main ores |
|
||
| Main products |
|
||
| applications |
|
||
| Occurrence | In the world |
|
|
| In Brazil |
|
Enem exercises and entrance exams
1. (Enem/2018) In Greek mythology, Niobia was the daughter of Tantalus, two characters known for their suffering. The chemical element with atomic number (Z) equal to 41 has chemical and physical properties so similar to the element with atomic number 73 that they were confused.
Therefore, in honor of these two characters in Greek mythology, these elements were given the names of niobium (Z = 41) and tantalum (Z = 73). These two chemical elements have acquired great economic importance in metallurgy, in the production of superconductors and in other high-end industry applications, precisely because of the chemical and physical properties common to both.
KEAN, S. The disappearing spoon: and other true stories of madness, love and death from chemical elements. Rio de Janeiro: Zahar, 2011 (adapted).
The economic and technological importance of these elements, due to the similarity of their chemical and physical properties, is due to
a) have electrons in the f sublevel.
b) being elements of internal transition.
c) belong to the same group on the periodic table.
d) have their outermost electrons at levels 4 and 5, respectively.
e) be located in the family of alkaline earth and alkaline, respectively.
Correct alternative: c) belong to the same group on the periodic table.
The periodic table is organized into 18 groups (families), where each group brings together chemical elements with similar properties.
These similarities happen because the elements of a group have the same number of electrons in the valence shell.
Doing the electronic distribution and adding the electrons from the most energetic sublevel to the outermost sublevel we find the group to which the two elements belong.
| Niobium | |
|
Distribution electronics |
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d3 |
|
sum of electrons |
more energetic + more external 4d3 + 5s2 = 5 electrons |
| Group | 5 |
| Tantalum | |
|
Distribution electronics |
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p66s2 4f145d3 |
|
sum of electrons |
more energetic + more external 5d3 + 6s2 = 5 electrons |
| Group | 5 |
The elements niobium and tantalum:
- They belong to the same group on the periodic table.
- They have their outermost electrons at levels 5 and 6, respectively, and so are located in the 5th and 6th period.
- They have electrons in the d sublevel and, therefore, they are transitional elements outside.
2. (IFPE/2018) Brazil is the world's largest producer of niobium, accounting for more than 90% of the reserve of this metal. Niobium, symbol Nb, is used in the production of special steels and is one of the most resistant metals to corrosion and extreme temperatures. The Nb compound2O5 it is the precursor of almost all alloys and niobium compounds. Tick the alternative with the required mass of Nb2O5 to obtain 465 grams of niobium. Given: Nb = 93 g/mol and O = 16 g/mol.
a) 275 g
b) 330 g
c) 930 g
d) 465 g
e) 665 g
Correct alternative: e) 665 g
The precursor compound of niobium is Nb oxide2O5 and the niobium used in the alloys is in the elemental form Nb.
Therefore, we have the following stoichiometric relationship:
1 mole of Nb2O5 generates 2 moles of Nb, as niobium oxide is formed by 2 atoms of this metal.
1st step: calculate the number of moles of niobium produced which corresponds to 465 g.
If by the calculation we saw that the mass of niobium corresponds to 5 moles, then the number of moles of Nb2O5 used is half of this value, because:
2nd step: calculate the molar mass of niobium oxide.
3rd step: calculate the mass of niobium oxide that corresponds to 2.5 mols.
3. (UECE/2015) Brazil holds 98% of the world's niobium reserves, which has numerous industrial applications, such as in jewelry manufacturing, hyperallergenic implants, electroceramics, superconducting magnets, magnetic resonance machines, metal alloys, special coins and in the production of steel. For niobium, review the statements below and tick the only true alternative.
a) Its differential electron is located in the penultimate shell.
b) It is a representative element.
c) Its electronegativity is lower than that of vanadium.
d) It belongs to the fourth period of the periodic table.
Correct alternative: a) Its differential electron is located in the penultimate shell.
When performing the electronic distribution of niobium, it is possible to see that its differential electron is located in the penultimate shell.
Because it has the differential electron in the d sublevel, it is an outer transition element.
Since its outermost level is in the fifth layer, niobium is located in the fifth period of the table.
Electronegativity is the property related to the element's ability to attract electrons and it varies according to the atomic radius: the smaller the atomic radius, the greater the attraction for electrons and, therefore, the greater the electronegativity.
Consulting the table with the electronegativity values, it is possible to see that niobium and vanadium have values close to 1.6 Pauling.
4. (UEA/2014) The natural isotope of niobium is the 93Nb. The number of neutrons in this isotope is
a) 41.
b) 52.
c) 93.
d) 134.
e) 144.
Correct alternative: b) 52.
Isotopes are atoms of a chemical element with different mass numbers.
Atomic mass corresponds to the sum of the protons and neutrons of an element.
The number of protons represents the atomic number of the chemical element and for isotopes it does not change.
Thus, the mass variation of isotopes occurs due to the different number of neutrons.
If the atomic number of niobium is 41, then the number of neutrons is given by the calculation:
5. (IFMG/2015) The chemical element niobium, Nb, is named after the Greek goddess Niobe. Brazil is the world's largest producer of the metal, accounting for 75% of production. Due to the thermal stability of its alloys, niobium is used in the production of special high-strength steel alloys for engines, propulsion equipment and various superconducting materials. Observing the position of niobium on the periodic table, it is correct to state that:
a) your most energetic sublevel will be sublevel d.
b) is an element belonging to the alkali metal family.
c) forms ionic compounds with other metals.
d) its cations will have an atomic radius greater than the pure element.
Correct alternative: a) your most energetic sublevel will be sublevel d.
By looking at the periodic table we can see that niobium is characterized as an outer transition element, which belongs to group 5 of the periodic table, as its most energetic sublevel is d.
We may also obtain this information by making electronic distribution.
As it is a metal, this element makes metallic connections with other metals, as in the alloy iron-niobium or also covalent bonds, by sharing electrons, as in niobium oxide Nb2O5.
6. (UFSC/2003) Niobium was discovered in 1801, by the English chemist Charles Hatchett. Brazil holds about 93% of the world production of niobium concentrate. The largest deposits are located in the states of Minas Gerais, Goiás and Amazonas. The metal is mainly used in the manufacture of iron-niobium alloys and other more complex alloys, which have been applied in the construction of jet propulsion turbines, rockets and spacecraft. Its oxides are used in the manufacture of light lenses for eyeglasses, photographic cameras and other optical equipment. Given (Z = 41). Regarding niobium, mark the CORRECT proposition(s).
(01) The niobium, when losing 3 electrons, assumes the configuration of krypton.
(02) Niobium can form M-type metal oxides2O5 in2O3.
(04) The chemical symbol for niobium is Ni.
(08) Niobium is a transition metal.
(16) An iron-niobium alloy is an example of a solid solution.
Correct alternatives: 02 + 08 + 16 = 26.
(01) INCORRECT.
| Elements | Eletronic distribution |
| 36Kr | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 |
|
41Nb 41Nb3+ |
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d3 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4p6 4d2 |
(02) CORRECT
Considering the oxidation numbers 3+ and 5+ for niobium, it can form the compounds:
| Oxidation number 5+ | Oxidation number 3+ |
| Nb2O5 | Nb2O3 |
(04) INCORRECT
Ni is the symbol for the element nickel. The symbol for niobium is Nb.
(08) CORRECT
Niobium is an external transition metal belonging to group 5 of the periodic table.
(16) CORRECT
A solid solution corresponds to a mixture of two or more components in the same phase, which is solid, being common among metals.
7. (UERJ/2013) Niobium is a metal found in natural deposits, mainly in the form of oxides.
In a deposit containing niobium with an oxidation number +5, the formula for the predominant oxide of this metal corresponds to:
a) NbO5
b) Nb5O
c) Nb5O2
d) Nb2O5
Correct alternative: d) Nb2O5
Oxygen makes two bonds and has a fixed oxidation number, which is 2-.
Therefore, to form niobium oxide, oxygen needs to bind to 2 atoms of this metal.
Niobium has different oxidation states. With oxidation number 3+ it binds to 3 oxygens and with Nox 5+ it forms the compound: Nb2O5 wherein 2 niobium atoms bond with 5 oxygen atoms.
Read the text to answer questions 8 to 10.
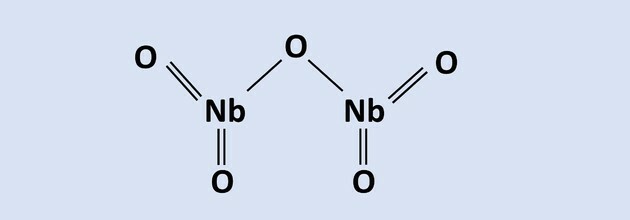
Niobium is a metal of great technological importance and its main world reserves are located in the
Brazil, in the form of pyrochlore ore, consisting of Nb2O5. In one of the processes of its extractive metallurgy, aluminothermia is used in the presence of Fe oxide2O3, resulting in an alloy of niobium and iron and aluminum oxide as a by-product. The reaction of this process is represented in the equation:
In nature, niobium appears in the form of the stable isotope niobium-93, but several unstable synthetic isotopes are known, which decay by emission of radiation. One of them is niobium-95 which decays to the element molybdenum-95.
(Systems.dnpm.gov.br; Tech. Metal. Mater. Miner., São Paulo, v. 6, no. 4, p. 185-191, Apr.-Jun. 2010 and G. Audi et al./Nuclear Physics A 729 (2003) 3–128. Adapted)
8. (FGV/2019) In the aluminothermia reaction to obtain the alloy of niobium and iron, considering the stoichiometry presented in the balanced equation, the total number of electrons involved in the process is
a) 6.
b) 12.
c) 18.
d) 24.
e) 36.
Correct alternative: e) 36.
The redox reaction occurs with the loss and gain of electrons.
When an element reduces it gains electrons and when an element is oxidized it loses electrons.
When an element reduces it is an oxidizing agent, whereas when an element oxidizes it is a reducing agent.
In this way, the number of electrons that were lost by one element and given up to another are equal.
| Element | NOX | Reaction | electrons | |
| Niobium |
+5 3Nb2O5 |
0 6Nb |
Reduction | 3.2.5 = 30 and- gains |
| Iron |
+3 Faith2O3 |
0 2Fe |
Reduction | 2.3 = 6 and- gains |
| Aluminum |
0 12Al |
+3 6Al2O3 |
Oxidation | 6.2.3 = 36 and- lost |
The charge of aluminum on the aluminum oxide product is 3+, that is, each aluminum has lost 3 electrons.
But in the products we have 12 aluminum atoms, which makes the total number of electrons involved in the process:
12. 3 = 36 electrons.
9. (FGV/2019) In an aluminothermic operation for the production of niobium and iron alloy with stoichiometric amounts of Nb2O5 and Fe2O3 and use of excess metallic aluminum, 6.12 tons of Al were formed.2O3. The sum total of the amounts, in moles, of niobium and iron estimated to be obtained in this operation is
a) 6 × 104
b) 6 × 106
c) 8 × 103
d) 8 × 104
e) 8 × 106
Correct alternative: d) 8 × 104.
1st step: calculate the molar mass of Al2O3
2nd step: calculate the number of moles of Al2O3
3rd step: perform the stoichiometric relationships.
In the chemical equation, we see that there is the relationship: 6 moles of niobium, 6 moles of aluminum and 2 moles of iron.
By the ratio of the numbers of formed mols, we have:
And the sum of the amounts of niobium and iron, in moles, is:
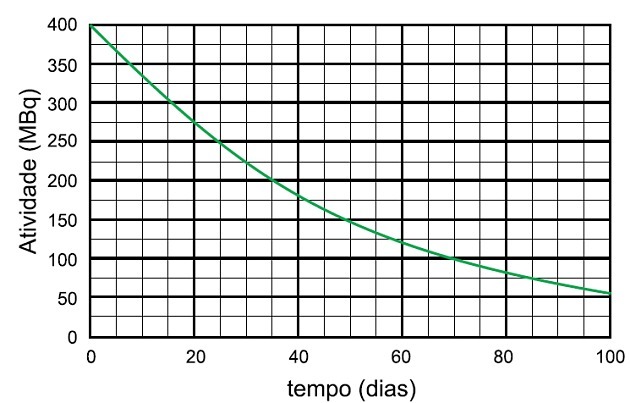
In the niobium-95 radioisotope decay process, the time taken for the activity of this sample to decay to 25 MBq and the name of the species emitted are
a) 140 days and neutrons.
b) 140 days and protons.
c) 120 days and protons.
d) 120 days and particles ß–.
e) 140 days and particles ß–.
Correct alternative: e) 140 days and particles ß–.
The half-life is the time it takes for a radioactive sample to halve its activity.
In the graph we see that the radioactive activity starts at 400 MBq, so the half-life is the time it took for the activity to decay to 200 MBq, which is half of the initial one.
We analyze in the graph that this time was 35 days.
For the activity to drop by half again, another 35 days passed and the activity went from 200 MBq to 100 MBq when another 35 days passed, that is, from 400 to 100 MBq 70 days passed.
For the sample to decay up to 25 MBq, 4 half-life times were required.
Which corresponds to:
4 x 35 days = 140 days
In radioactive decay, emissions can be alpha, beta or gamma.
Gamma radiation is an electromagnetic wave.
Alpha emission has a positive charge and decreases 4 units in mass and 2 units in the atomic number of the decayed element, turning it into another element.
Beta emission is a high-speed electron that increases the atomic number of the decayed element by one unit, turning it into another element.
Niobium-95 and molybdenum-95 have the same mass so a beta emission occurred because: