Halogen lamps (also called halogen lamps) are widely used by professionals in architecture and interior design to create different environments that require more lighting points intense.
These lamps have the same operating principle as incandescent lamps, that is, they are generally formed by an ampoule with a thin filament of tungsten (W), metal with a very high melting point, withstanding high temperatures. When this tungsten filament is traversed by electrical current, it emits a white light with a slightly yellowish tinge.
Over time, the tungsten sublimes (changes from a solid to a gaseous state) from the filament and deposits in the bulb, darkening the bulb:
W(s) ↔ W(g)
However, the difference between common incandescent lamps and halogen lamps is that the latter contain gaseous iodine inside. Iodine is from the family of halogens in the periodic table, hence the name given to this type of lamp.
In the filament, the iodine reacts with the gaseous tungsten that is released, forming the tungsten iodide gas:
W(g) + 3 I2(g) ↔ WI6 (g)
When this gas approaches the bulb, which is a cooler region, it decomposes, thus recovering the metallic tungsten (solid), which is again deposited on the filament.
WI6 (g) ↔ W(s) + 3 I2(g)
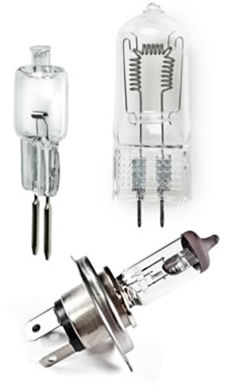
The result is that this type of lamp does not dim and lasts longer. Ordinary incandescent lamps last an average of 1 year or 1000 hours, while halogen lamps last 2000 hours and can reach 5000 hours. They are also a little more efficient or economical than incandescent ones. But, it is noteworthy that they consume more energy compared to fluorescent or discharge ones.
These points and the fact that its light is brighter than the common incandescent is what makes its use to be growing more and more.
One type of halogen lamp is the dichroic lamp, which has a reflector that reduces the excessive heat produced.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/equilibrio-quimico-lampadas-halogenas.htm
