When we say the word “balance” an object that remains indefinitely comes to mind. However, this is just one kind of balance, called “static equilibrium”.
There is also the “dynamic equilibrium”. In it, as the name says, there is not a single moment in which the object or phenomenon in question is still. For example, for you to understand, see the illustration below and note that the amount of water that falls into the container is equal to the amount that flows out of it, keeping the water level constant. In that case, we say that there is a dynamic balance, a balance in motion.

It's this kind of balance that occurs in reversible reactions, that is, in those reactions that occur in both directions. At the same time that the molecules of the reactants are transformed into the products, the molecules of the products react with each other to form the reactants. The reversibility of a reaction is represented by arrows in both directions:
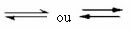
When the rate of development of the direct reaction (of formation of the products) is equal to the rate of development of the inverse reaction (formation of reactants), under constant temperature, means that the reaction has reached its equilibrium chemical. And in the case of reactions with the presence only of molecules in the reactants and products, we have a molecular balance.
The following is an example of the reaction that occurs between hydrogen gas (H2) and iodine gas (I2), for the formation of hydrogen iodide (HI) gas:
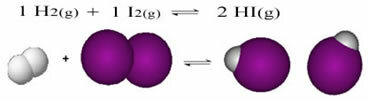
At the beginning of the reaction, the rate of development of the direct reaction was higher, after all the concentration of reactants was maximum and that of the products was zero. So the rate of development of the reverse reaction was zero too.
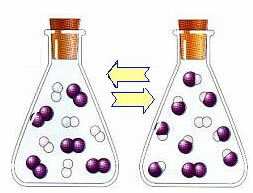
However, over time, the hydrogen and iodine gases react, generating the product. In this way, the concentration of reagents starts to decrease and their rate of development also decreases.
As the concentration of products increases and the concentration of reactants decreases, the rate of development of the inverse reaction starts to increase. If the temperature is kept constant, there will come a time when the two rates of development will remain the same, thus showing that the reaction has reached chemical molecular equilibrium.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/equilibrio-molecular.htm

