As explained in the text "Phlogiston Theory”, it was long believed that this theory provided the explanation for the phenomenon of combustion. She said that combustible materials had a common flammable principle present only in them, which became known as phlogiston. If some material didn't burn, it's because it wouldn't have phlogiston in its composition.
However, some scientists began to disagree with this conclusion, as there are also several contradictions in this theory, the experiments carried out brought other evidence, which did not exist before, that led these studies to another direction.
One scientist who excelled in these combustion studies was Antoine Laurent Lavoisier (1743-1794). One of his most famous experiments was to place, in a retort, a carefully weighed sample of metallic mercury and introduce the retort tube into a glass dome or vat containing air and also mercury in its base.
He heated this retort with mercury through an oven, causing it to burn. Lavoisier observed that as the reaction proceeded, a red powder, mercury oxide II, formed on the walls of the retort.
the volume of mercury in the vat was rising. This meant that the air volume was decreasing as it was being replaced by mercury., as seen in the figure below. In weighing the initial and final system, Lavoisier saw that the mass had not changed.
Thus, Lavoisier concluded that combustion did not occur because of the presence of a mysterious phlogiston, but yes because mercury or any other combustible material reacted with another element present in the air.
At the same time, English scientist Joseph Priestley showed Lavoisier that he had discovered a kind of “air”, which he called "dephlogisticated air". Through his own experiments, Lavoisier was able to produce this air and carried out other experiments with it.
For example, he placed a glass vat over a lit candle in a buoy of water. He noticed that as the candle went out, the water rose. And when the water reached a fifth of its volume, the candle went out completely. The conclusion was as follows:
(1st) The water rose because the candle was consuming the air;
(2nd) The “dephlogisticated air” was not the entire atmospheric air, but the fifth part of it.
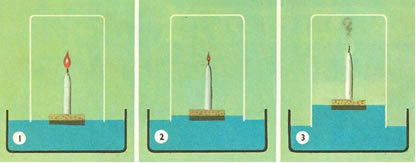
Thus, Lavoisier found that this air was mixed with all atmospheric air and that it was necessary for combustion; without it combustion did not take place. Lavoisier was even the first to make an experimental determination of the composition of the air, arriving at the result of 21% oxygen and 79% of another component, which he called nitrogen, a “type of air” that did not participate in the combustion. Today we know it was nitrogen gas.
Initially he called the dephlogisticated air the "breathable air" and then changed to "vital air".It was only in 1778 that Lavoisier decided to name the “vital air” oxygen (a word that comes from the Greek oxy, which means “acid”; and gen, “generator or product”). He gave it this name because until then his experiments had led him to the conclusion that this new gas was present in all acids; what later turned out to be a wrong conclusion, the name still stuck.
Until then, oxygen was not considered a chemical element as we know it today, because at that time there was still no concise definition for an element.
Carl Wilhelm Scheele was the first to isolate oxygen, however, he did not see the importance of the discovery that he reached as it was still very much linked to the phlogiston theory. It was Lavoisier who interpreted and showed the role of oxygen in combustion.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/descoberta-oxigenio.htm
