Carbon chains represent the structure of organic compounds.
They get that name because they form from the bonding of carbon atoms.
There are several types of chains and their classification is done according to the position of the carbon atoms, the bond between them, the bond between hydrogen atoms or other compounds.
Classification
The criteria for classifying carbon chains are:
- Closing the chain or not
- Types of atoms, with or without heteroatoms (atoms that are not carbon or hydrogen)
- Organization of chain atoms
- Bonds established between atoms
They can be open, closed or mixed:
1. open chains
They are also called acyclic and aliphatic. In this type of chain, the carbon atoms link and leave the ends free.
Normal, straight or linear open chains:
In linear open chains, no branches are formed.
Open branched chains:
Open branched chains have branches.
Open homogeneous chains:
Homogeneous chains have only one carbon atom.
They have no heteroatom, that is, no atom other than carbon or hydrogen in the chain.
Heterogeneous open chains:
Heterogeneous chains have at least one atom other than carbon or hydrogen along the chain.
Saturated open chains:
In saturated chains the carbon atoms are linked together by a single bond. The carbon in this chain is called saturated.
Unsaturated open chains:
These are chains that have at least two carbon atoms joined by a double to a triple bond. In this bond, carbon is called unsaturated.
2. closed chains
Closed or cyclic chains link together and form a cycle.
They can be aromatic or alicyclic. If they are alicyclic, they are further classified as homocyclic, heterocyclic, saturated or unsaturated.
Closed aromatic chains:
Closed aromatic chains are classified into:
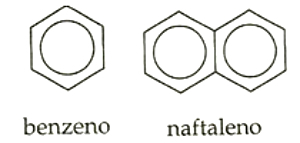
- Mononuclear: when they have only one aromatic ring, such as benzene.
- Polynuclear: when they have more than one aromatic ring, such as naphthalene.
Closed alicyclic chains:
Closed alicyclic chains do not exhibit aromatic rings. They are divided into saturated and unsaturated.
Unsaturated alicyclic closed chains are divided into:
- Homogeneous: The ring of these chains is formed only by carbon atoms.
- heterogeneous: These are chains that have a heteroatom.
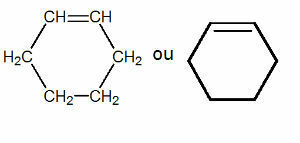
Saturated closed chains:
In a saturated chain all carbon atoms make only single bonds.
Unsaturated closed chains:
In the unsaturated chain there are double bonds between atoms.

3. Mixed Chains
In mixed chains, the carbons bond together and, as with closed chains, they also form a cycle in the chain.

Read too:
- Aromatic hydrocarbons
- Nomenclature of hydrocarbons
- Organic chemistry
- Carbon
Chain carbon classification
Carbon can also be classified according to its position in the chain into:
primary carbon: are the atoms at the ends of the chains and which only bind to another atom.

secondary carbon: is the carbon that binds to two other carbon atoms in the chain.
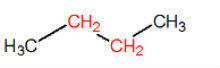
tertiary carbon: when carbon binds to three other carbon atoms.

Quaternary carbon: when carbon appears attached to four other carbon atoms.

Read too:
- Benzene
- Hydrocarbons
- Organic compounds
- Exercises on Organic Chemistry
- Exercises on Organic Functions