Chlorine is a chemical element with the symbol Cl, atomic number 17, atomic mass 35.5. It belongs to the halogen family, group 17 or 7A, and to the third period of the periodic table.
Its name derives from the Greek khloros, which means greenish. This is because under normal conditions of temperature and pressure, chlorine is characterized as a greenish-yellow gas with a strong smell.
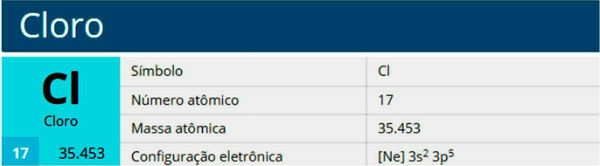
Features
Chlorine was discovered in 1774 by the Swedish scientist Carl Wilhelm Scheele (1742-1786). However, at that time he believed that it was a compound with oxygen. In 1810, Humphry Davy (1778-1829) demonstrated that it was a new chemical element.
As it is an extremely reactive element, it is hardly found in nature in its pure form, with the exception of the small amount emitted during volcanic eruptions in the form of HCl.
Thus, it is commonly found in the form of sodium chloride (NaCl), also known as table salt. In minerals, it occurs in the form of carnallite and sylvite.
It can also be obtained by electrolysis of NaCl, in aqueous solution. Chlorine also produces many salts from chlorides through the process of oxidation.
Learn more, read also:
- Periodic table
- Chemical elements
- Halogens
applications
Chlorine gas (Cl2) is toxic and irritating, this condition led to it being used as a chemical weapon during the 1st World War. This gas causes irritation in the respiratory tract and skin, water retention in the lungs, tearing and when inhaled in large quantities can lead to death.
Some other uses of chlorine are:
- Bleaching of paper and fabrics using chlorine dioxide (ClO2).
- Water treatment, the addition of chlorine makes the water drinkable and fit for human consumption. This process is called chlorination and uses hypochlorous acid (HClO).
- Disinfection of swimming pool water and industrial waste, as chlorine is capable of killing microorganisms.
- Production of plastic compounds such as PVC (polyvinyl chloride) and synthetic rubber.
- Production of some types of organic and inorganic compounds.


