Geometric electronic distribution is a different way of representing a fundamental electronic distribution of an atom, that is, the one commonly performed with the Linus Pauling energy diagram.
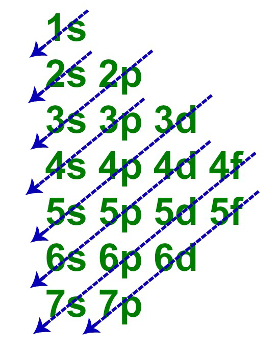
Linus Pauling diagram in which the arrows indicate the order of energy
Therefore, it is necessary to emphasize that the construction of the geometric electronic distribution depends on the realization of the fundamental distribution. The differences between them are:
The geometric distribution is always represented on a single line;
The geometric distribution does not follow the order (blue arrows) of energy, as performed by the Linus Pauling diagram;
The fundamental distribution can be represented in a single line, however, it must always follow the order of energy in the diagram.
To understand how a geometric electronic distribution, let's use the element zinc (atomic number 30) as an example from the following steps:
1st Step: distribute the thirty electrons referring to the atomic number.
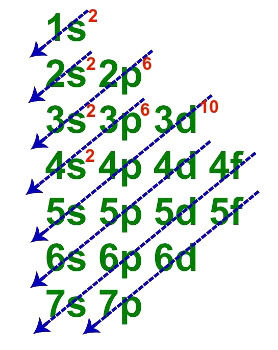
Zinc fundamental electronic distribution
2nd Step: Make the geometric distribution in a line representing each of the lines (1, 2, 3 and 4) of the diagram:
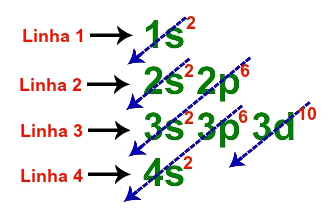
Lines used in fundamental distribution of zinc
The four lines of the fundamental distribution of zinc are represented in the geometric distribution as follows:
Zinc geometric distribution: 1s2 2s2 2p6 3s2 3p6 3d10 4s2
Features of geometric electronic distribution
Determine the outermost sublevel of the atom;
Determine the location of electron withdrawal in an atom with a tendency to form cation;
Determine the location of electron withdrawal in an atom with a tendency to form anion.
Examples of geometric electronic distributions
1st Example: Uranium element (Z=92)
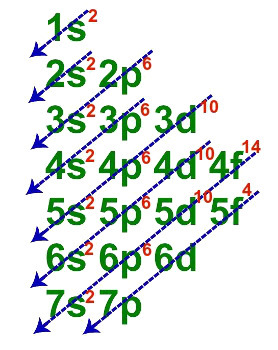
Electronic distribution of the element uranium
The fundamental electronic distribution of uranium has a total of seven lines, as in the following description:
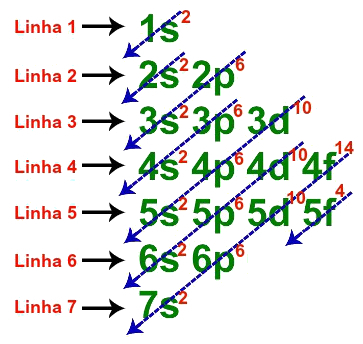
Lines used in the fundamental distribution of uranium
When each of the seven lines is represented in a single one, the electronic geometric distribution of uranium is presented as follows:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f4 6s2 6p6
2nd Example: Cesium element (Z = 55)
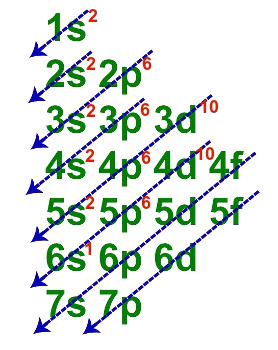
Electronic distribution of the element cesium
The fundamental electronic distribution of cesium has a total of seven lines, as in the following description:
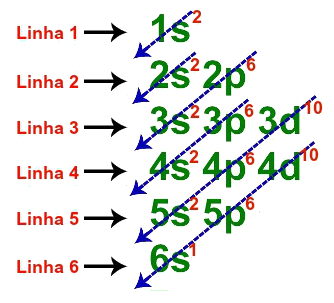
Lines used in the fundamental distribution of cesium
When each of the six lines is represented in a single one, the electronic geometric distribution of cesium is presented as follows:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-distribuicao-eletronica-geometrica.htm

