Thermometric scales are used to represent the temperature in different units. Every thermometric scale has two fixed points which are defined from some physical property of some substance that can be easily observed when the body is subjected to the same conditions.
Celsius scale
The most widely used thermometric scale in the world is the Celsius scale. This scale is based on pure water and has as fixed points the melting points (0°C) and boiling water (100°C), under normal pressure conditions. The Celsius scale was created in the year 1722, by the Swedish astronomer AndersCelsius.
See too: How do heat exchanges take place?
Fahrenheit scale
The Fahrenheit scale was discovered by DanielGabrielFahrenheit, a German physicist and engineer, in the year 1724.
Some countries like the United States still use the Fahrenheit scale. This scale was created so that the melting temperature of water was 32°F and that every degree of that scale corresponds to 1.8 degrees on the Celsius scale, so the melting temperature of water, on the Fahrenheit scale, is 212°F.
Both scales, Celsius and Fahrenheit, they are considered usual scales, that is, they are not thermodynamic scales. A thermodynamic scale must take into account the fact that its lowest temperature value, the one at which the atoms of a portion of substance are found perfectly at rest, should be equal to 0, unlike what occurs on the scales cited, that admitvaluesnegatives of temperature.
An important difference between the Fahrenheit scale and other scales is that this it's not a centigrade scale, that is: the distance between your fixed points is not 100 degrees, but 180 degrees.
Kelvin scale
The first scale that took into account the non-use of negative values of temperature was the Kelvin scale, currently adopted by the International System of Units. The Kelvin scale attributes temperature to the thermal agitation of atoms, so its lower limit is 0 K, known as absolute zero. For historical and practical reasons, some countries continue to use the Celsius and Fahrenheit scales.
The Kelvin scale was proposed in 1848 by William Thomson, an Irish physicist and engineer known by the title Lord Kelvin. Unlike other temperature measurements, the Kelvin scale does not use the terminology of degrees, as it is an absolute unit of temperature and not a scale created based on some substance.
Observe the image below, in it we have three thermometers calibrated on the Kelvin, Celsius and Fahrenheit scales, respectively, which show the fixed points of each of the three mentioned scales:

Calibrated thermometers on Kelvin, Celsius and Fahrenheit scales
Conversion of thermometric scales
It is possible to convert temperature values between the three most common thermometric scales. For this, we use the following identity:
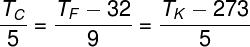
Check out an example of using the formula above. Let's convert the temperature from 80ºF to ºC:

Temperature variation in thermometric scales
Unlike the calculation that is done to perform the temperature conversion between scales thermometrics, we can calculate how much the temperature variation in one scale corresponds to another scale. For that, we can use the following equality:

According to the identities shown above, the temperature variation in degrees Celsius is the same as the variation suffered on the Kelvin scale, so if a body had a temperature increase of 10 °C, its temperature increased by 10K. However, if a body has experienced an increase of 10°C or 10K in its temperature, on the Fahrenheit scale its temperature will have increased by 18°F.
Exercises on thermometric scales
1) A thermometer measures a temperature equal to 20°F, determine the value of that temperature on the Celsius and Kelvin scale.
Resolution:
Let's use the equality between the thermometric scales to calculate the temperature of 20°F on the requested scales:

2) Determine what temperature so that the value is equal in Celsius and Fahrenheit.