Allotropy is a phenomenon that occurs when the same chemical element has the property of forming more than one simple substance.. These allotropic varieties may differ due to the number of atoms of the element that are bound together in a molecule or due to the arrangement of atoms in the crystal lattice.
One of the elements that have allotropic varieties is phosphorus (P), the most common being the white phosphorus it's the red phosphorus. These two phosphorus allotropes differ not by their different spatial arrangement, as both are basically made up of tetrahedral molecules, but the amount of phosphorus atoms is different in each one.
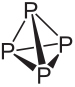
See the constitution and main characteristics of white and red phosphorus:
- White Phosphorus:
The molecular formula of white phosphorus is P4, which means that its molecules are made up of four phosphorus atoms.

This type of phosphor is extremely reactive, largely due to the tension at the 60° angles between its bonds. It's so reactive that it needs to be stored in water so it doesn't come into contact with the air and explode. Great care must be taken when handling it, as it causes severe skin burns and poisoning if swallowed (just 0.1 g of white phosphorus ingested can lead to death).
As you can see below, white phosphorus is a white wax-like solid.

White phosphorus stored in water so as not to come into contact with air*
If heated in the absence of air, white phosphorus turns into red phosphorus.
- red phosphorus:
Red phosphorus does not have a determined structure, but there is evidence that it is macromolecules formed by the binding of the tetrahedral structures mentioned (P4), being represented by Pno.
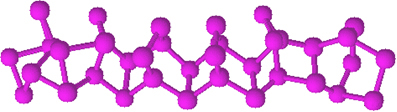
It is much less reactive than white phosphorus, however, it can ignite if rubbed. That's why it is used on the side surfaces of matchboxes. When we rub the toothpick on the surface of the box, the match ignites and, in turn, ignites the highly flammable material on the toothpick's head.
In some countries, the match is placed on the head of the toothpick in the form of P.4s3.
Red phosphorus is an amorphous powder, that is, it does not have a crystalline structure, and is dark red in color, as shown below:

Red Phosphorus Powder.
* Image authorship belongs to W. Oelen and she can be found on here.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/alotropia-fosforo.htm