You oxides they are made up of two different elements, one of which is oxygen, which needs to be the most electronegative of the two.
The nomenclature of inorganic oxides follows rules that depend on whether the oxide is molecular, covalent or ionic. Let's look at each case:
* Nomenclature of molecular oxides or covalent network:
Molecular or covalent network oxides are those that have oxygen attached to a ametal, such as carbon (C), nitrogen (N), sulfur (S), fluorine (F), among others. The nomenclature of these oxides follows the following rule:

Nomenclature rule for oxides formed with ametals
The mono prefix in front of the oxygen-linked element is optional.
For example, we have the following molecular oxide: CO.
- Prefix indicating the amount of oxygen: 1 oxygen: mono;
- oxide of;
- Prefix that indicates the number of atoms of the other element: 1 carbon: mono;
- Name of the element linked to oxygen: carbon.
So, your name is like this: CO = monocarbon monoxide or carbon monoxide.
See more examples:
carbon dioxide - CO2
Sulfur trioxide - SO3
Dichloro Heptoxide - Cl2O7
Dinitrogen Monoxide - N2O
Dinitrogen trioxide - N2O3
Nitrogen Monoxide -NO
Nitrogen dioxide - NO2
Dinitrogen pentoxide - N2O5
Silicon dioxide - SiO2
Diphosphorus pentoxide - P2O5
Sulfur trioxide - SO3
* Nomenclature of ionic oxides:
Ionic oxides are those that have oxygen bound to a metal, such as iron (Fe), lead (Pb), sodium (Na), calcium (Ca), silver (Ag), among others. Generally, the electrical charge of oxygen is -2.
The nomenclature mentioned for molecular or covalent network oxides is also currently applied to metallic oxides and is considered as official. See some examples:
Iron Monoxide - FeO
Lead Monoxide - PbO
Lead Dioxide - PbO2
Diferous trioxide - Fe2O3
However, there is a specific nomenclature for metal oxides that is still widely used. It is based on the valence of the element linked to oxygen.
If the element has a single valence, that is, if there is only one way to bind oxygen and form only one type of oxide, the naming rule will be given by:

Single-valent ionic oxide naming rule
Examples:
- Sodium oxide - Na2O
- Calcium Oxide - CaO
- Potassium oxide - K2O
- Aluminum Oxide - Al2O3
- Silver oxide - Ag2O
But there are also ionic oxides formed by elements with more than one valence. In these cases, the naming rule is as follows:
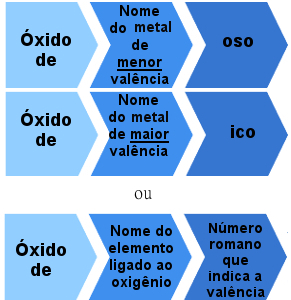
Nomenclature rule for ionic oxides with more than one valence
Examples:
Ferrous Oxide - FeO
Ferric Oxide - Fe2O3
Cuprous Oxide - Cu2O
cupric oxide - CuO
Or:
Iron oxide II - FeO (Iron nox = +2)
Iron oxide III - Fe2O3 (Iron nox = +3).
Copper Oxide I - Cu2O (Iron nox = +1)
Copper oxide II - CuO (Iron nox = +2).
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nomenclatura-dos-oxidos.htm