Organic compounds are molecular, that is, their atoms carry covalent bonds with each other. When we analyze the bonds between carbons, which can be single, double or triple, we observe that they are nonpolar bonds, as there is no difference in electronegativity between the atoms, as they belong to the same element.
Furthermore, since hydrogen and carbon have a very small electronegativity difference, the bonds between them are also non-polar.
Non-polar connections:
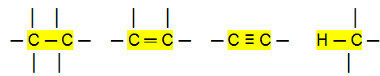
With that, we can conclude that the Hydrocarbons (organic compounds that have only carbon and hydrogen atoms) are non-polar molecules. In these compounds, the intermolecular interaction is of the induced dipole type, which is the weakest that exists.
Since they are weak, these interactions are easy to break. Due to this, the boiling and melting temperatures of hydrocarbons are lower than those of other functions.

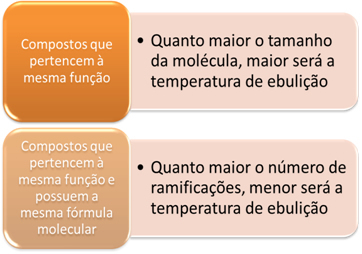
Comparing hydrocarbons, boiling points will increase as molar mass also increases.
For example, ethane and butane are both alkanes. See the boiling points of each experimentally determined:
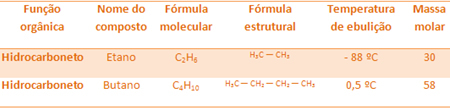
Note that the boiling temperature of butane is much higher than that of ethane, as its molar mass is also higher.
Now when we compare hydrocarbons that have the same molar mass (they are isomers), but that have different types of carbon chains, we realize that the greater the number of branches, the lower the boiling temperature, because the structure of the molecule becomes more compact, that is, its surface decreases.
All alkanes below have the same molecular formula, C5H12, but their boiling temperatures are different:
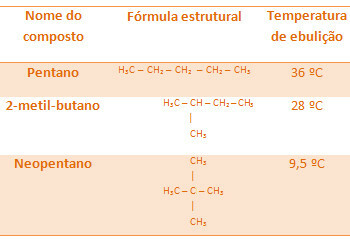
Note that neopentane's boiling temperature is the lowest as it has more branches.
We can consider that the other organic functions are derived from hydrocarbons, through the replacement of one or more hydrogens by atoms or groups of atoms of other elements. Generally, the other organic functions have oxygen or nitrogen, which are more electronegative elements than carbon. They more strongly attract the electron pair shared with carbon and, therefore, make the molecule polar:
Polar connections:
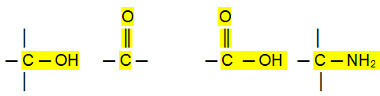
Aldehydes, ketones and organic halides have higher boiling points than hydrocarbons, because their intermolecular interaction is the permanent dipole, which is stronger than that of induced dipole.
Alcohols, carboxylic acids and amines, on the other hand, have even higher boiling temperatures, as they carry out hydrogen bonds, the most intense type of intermolecular interaction.
In compounds of all these functions, the same applies as we saw for hydrocarbons:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/polaridade-temperatura-ebulicao-dos-compostos-organicos.htm
