Chlorides are those ionic compounds that contain the anion Cℓ-1.
Chlorine is of the 17 or 7A family, its atomic number is equal to 17 and it has 7 electrons in the valence shell (the outermost electron shell of the atom). According to rule of the octet, to be stable, it needs to have 8 electrons in this last shell and therefore needs to receive one more electron. When this happens, that is, when it receives an electron, in general, from an ionic bond with a metal, the â is formed.nion chloride (Cℓ-1), the metal that gave up the electron becomes a cation and the substance formed is ionic.
Chlorides are salts derived from the reaction of a base with hydrochloric acid (HCℓ(here)). The base provides the cation and hydrochloric acid provides the chloride ion:
Generic base + hydrochloric acid → Chloride + Water
ÇOH+HCℓ → ÇCℓ + H2O
Chlorides are all classified as inorganic salts, because in an aqueous medium they release a cation other than H+ and release the chloride anion, not the hydroxyl (OH-).
The nomenclature of chlorides always follows this rule: Chloride +de + (name of element linked to chlorine). At your formulas are formed exchanging the ion charges for the indices (number that is at the bottom right of the element symbol, indicating the number of atoms of that element that are present making bonds).
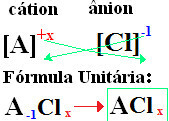
if the index is "1", you do not need to write it in the formula, as shown below:
At+1 Cℓ-1 → At1Cℓ1 → AtCℓ: sodium chloride;
K+1 Cℓ-1 → KCℓ: potassium chloride;
Here+2 Cℓ-1→CaCℓ2: calcium chloride;
Ba+2 Cℓ-1→Bhere2: barium chloride;
Aℓ+3 Cℓ-1→ACℓ3: aluminum chloride.
However, as explained in the text “Ionic substances of the group: chloride, carbonate, nitrate and sulfate”, as with all ionic substances, chlorides do not have their unitary formulas isolated in nature, as their ions attract each other mutually and form crystal lattices.
The main example of chloride that we have in our daily lives is the sodium chloride, NaCℓ, the table salt. See the ionic bond that results in its formation (in which sodium donates an electron to chlorine) and below the crystal lattice of this salt:
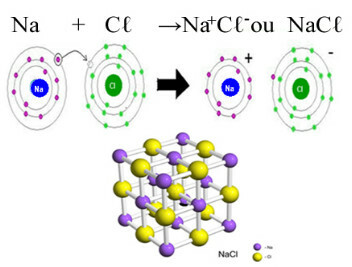
In the crystalline lattice of sodium chloride each anion Cℓ- is surrounded by 6 cations Na+ and vice versa, so the coordination number of this crystal lattice is 6.
All chlorides are solids in ambient conditions and quite water soluble, being among the most common salts found in our daily lives, considering that several of them are present dissolved in mineral water, drinking water, tap water, rivers, seas, among others. Among the exceptions, being water-insoluble chlorides, are silver chloride (AgCℓ - shown in the following figure), from lead, copper and mercury compounds.

Water-insoluble silver chloride*
The Cℓ ions-1 from the chlorides we ingest, mainly in the salt, fish and meat, are important for some functions of the human body, as they are the main extracellular anion, are present in the juice gastric, regulate bodily fluids, such as water distribution in the body, and maintain plasma osmotic pressure and neutrality electric.
Its deficiency can cause anxiety, diarrhea and circulatory problems. Its excess is excreted through urine (on average, each person excretes about 4 g of chloride per day), sweat and feces.
*Image Credit: Author: Danny S. / Image extracted from: Wikimedia Commons
By Jennifer Fogaça
Graduated in Chemistry
