THE density is one of the physical properties of matter (everything that occupies space in space and has mass). Other examples of physical properties are fusion point, boiling point and solubility.
At physical properties they are characteristics determined through the study of a material, without it being transformed into another.
In the specific case of density, its determination depends on the knowledge of two very important variables, the Weight (represented by the letter P) and the volume (represented by the letter V).
The weight of the material is determined by a scale, and the volume is usually measured using a beaker or beaker.

The beaker and scale are instruments used to determine, respectively, mass and volume
In the definition, density is the relationship between the weight and volume occupied by a matter. With this relationship, we can build the formula represented below:
d = P
V
Furthermore, it is not correct to say that we use the mass of material, as all matter on our planet is subjected to the action of gravity. Therefore, the term weight involves the multiplication between the mass and the gravity.
If we want to know the density of water, for example, we should measure any volume of this substance (50 mL) and then weigh it (50 g) on the scale (discounting the weight of the container, of course). Finally, we must perform the following calculation:
d = 50
50
d = 1 g/ml
Surely you might be wondering why knowing the density of a material is important, isn't it?
The answer is simple. Knowing the density is important because it is the property used, for example, to explain why a matter float in another, when both are liquid, or when one is solid and the other is liquid.
An example is when we put a certain amount of water with an amount of oil in the same container. The oil will float on the water, as shown in the following image:

Result of the mixture formed by water and oil in a container
The explanation for oil floating in water involves two aspects: the first is that it does not dissolve in water and the second is that it has a lower density than water. So, whenever we compare the density between materials, it is also important to check the solubility, which is the property that one material has in dissolving another.
We can also change the density of a material by dissolving another in it, as when we dissolve sodium chloride in water. When one material is dissolved in another, the tendency is for the density to increase. See the case below:
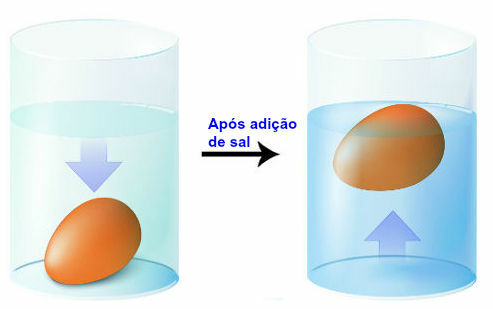
Modification of liquid density
When placed in water, a chicken egg sinks because its density is greater than that of liquid. If we dissolve sodium chloride in water, the density of that liquid increases, consequently the egg must float.
Another interesting curiosity is that the change in temperature it can also change the density of a matter, as in the case of air, for example. That's why the air conditioning equipment is placed on top of the wall, precisely because cold air has a higher density than hot air. Thus, cold air goes down and hot air goes up, which makes it possible to better cool the environment.

Air conditioner positioned high up
THE change in physical state The material also modifies the density, and this can be easily observed by adding a few ice cubes to a glass of water. In this case, water in the solid state (ice) is less dense than water in the liquid state.

ice floating in liquid water



