The Periodic Table is a way of organizing all chemical elements according to their properties and showing some information about them.
In everyday life, organization is very important to make our lives easier. For example, imagine your closet messy, with socks mixed in with shirts and pants. It would be very difficult and it would take longer to find a specific sock you wanted to wear, wouldn't you say?! But if you organize your wardrobe and put all your socks in one drawer, have a drawer. for t-shirts, another for shorts and so on, it will be much easier to find what need. And the more clothes you own, the more organization is needed.
Likewise, scientists have been discovering many chemical elements over time. To give you an idea, in 1850, around 60 elements were known, but today we know of the existence of 118. Thus, the need arose to organize these chemical elements in a way that would make it easier to understand their properties. This is the role of the Periodic Table of the chemical elements.
The Periodic Table we use today is arranged in horizontal lines in ascending order of atomic number.
You should consult the Periodic Table as if you were reading a normal text, that is, you always starts with the first row and from the left side to the right and then continues down to the next lines.The chemical elements were placed on the Periodic Table in separate squares, where the element symbol is in the half and the value of the atomic number is usually written at the top, as shown in the hydrogen example below:

Hydrogen symbol and its atomic number as shown in the Periodic Table
The atomic number is the amount of protons or positive charges the element's atoms have. This value is equal to the number of electrons when the atom is in its ground state.Hydrogen is an element that only has 1 proton, that is, its atomic number is 1. Therefore, hydrogen is the first element placed in the Table. The next element that's on the same line as hydrogen is helium, because it has an atomic number equal to 2.
Moving to the bottom row, the first is Lithium with an atomic number equal to 3, next to it is beryllium with atomic number 4 and so on.Look at the first lines of the Periodic Table shown below and see that the order of the atomic number is growing just right.
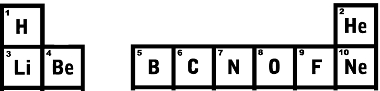
First two lines of the Periodic Table
There are seven lines in the Periodic Table and these lines are called periods. Look:

Periodic Table Periods
There are 18 columns that are called families or groups. An important aspect is that elements that belong to the same family are those that have similar physical and chemical properties.

Periodic Table Families or Groups
Let's see if you understand? Tell me which chemical element belongs to the 4th period and family 16?
If you said Se (selenium), you're right! Now tell me what his atomic number is. That's right, it's 34.
In each small square that the element sees there are also other important information, such as the atomic mass and the electrons that are in every electronic layer of atoms. For example, see in the following image that neon has Ne as a symbol, its atomic number is equal to 10, its atomic mass is equal to 20.1797 u and its electrons are distributed like this in their layers: 2 - 8, that is, in the layer closest to the nucleus there are two electrons and in the furthest one there are eight electrons.

Neon symbol on the Periodic Table and its atom
Now note two interesting aspects: (1) the neon only has two orbits or layers where your electrons are, that's why he occupies the 2nd period (2nd line);and (2) he has eight electrons on the last layer, that's why he is family 18.
This shows us the following:
* The elements that are in the same period of the periodic table have the same amount of electronic layers, and they can have a maximum of seven;
* Chemical elements that are in the same family on the periodic table have the same number of electrons in the last electron shell:
*Family 1: have all 1 electron in the last electronic layer;
*Family 2: have all 2 electrons in the last electronic layer;
*Family 13: have all 3 electrons in the last electronic layer;
*Family 14: have all 4 electrons in the last electronic layer;
*Family 15: have all 5 electrons in the last electronic layer;
*Family 16: have all 6 electrons in the last electronic layer;
*Family 17: have all 7 electrons in the last electronic layer;
*Family 18: have all 8 electrons in the last electronic layer.
Some groups or families in the Periodic Table are given specific names, see some:
Family 1: Alkali metals;
Family 2: Alkaline earth metals;
Family 16: Chalcogens;
Family 17: Halogens;
Family 18: Noble Gases.

Organization of Periodic Table Families
Again let's test your knowledge. Answer the following questions just by consulting the Periodic Table:
1- What is the name of the chlorine family?
2- What is its atomic number and its atomic mass?
3- How many electronic layers does a chlorine atom have?
4- How many electrons does a chlorine atom have in its last electron shell?
Answers:
1- Halogens (family 17).
2- The atomic number of chlorine is 17 and its atomic mass is equal to 35.45 u.
3- A chlorine atom has three electronic layers because it belongs to the 3rd period of the Table.
4- A chlorine atom has seven electrons in the valence shell because it belongs to family 17.
There is still other important information that the Periodic Table conveys to us and that we will talk about better in later texts. But the ones discussed here are the main ones for you to begin to understand how the elements are organized in it. Remember that a table is not made for decorating, but you must know it well to be able to refer to it when necessary.
By Jennifer Fogaça
Graduated in Chemistry
Take the opportunity to check out our video classes related to the subject: