The different physical states of Watercan be observed, of course, in our planet. The water in rivers and seas, for example, is in a liquid state. Glacier water is in solid state, and in atmosphere, we find water in a gaseous state. This substance can often change from one physical state to another depending on factors such as temperature and pressure.
Read more: Earth: water planet? Could we name our planet any other way?
The three physical states of water
Water is found in three physical states: solid, liquid and gas. It is one of the few substances that can be found naturally in its three states.
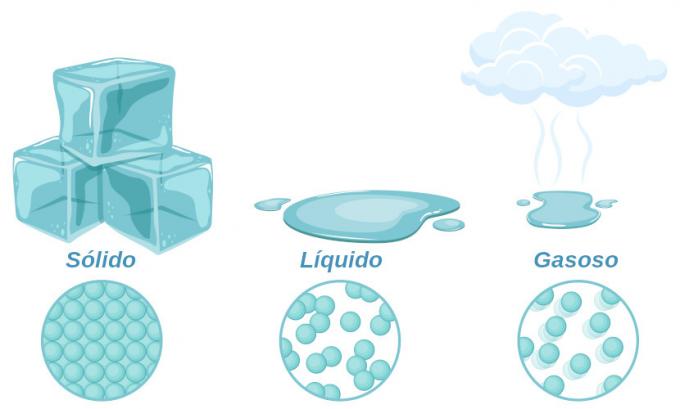
- Solid: solid state water can be observed, for example, in the poles, at the peak of mountains and on icebergs. In this case, the water molecules are very close to each other, and the substance acquires a well-defined shape and volume.
- Liquid: liquid water is found in rivers, lakes, seas, oceans and groundwater. This is the main way water is found on the planet. Water in the liquid state has molecules further apart than in the solid state, and it takes on the shape of the container in which it is contained. You can check this property by looking at water in a glass. When in the liquid state, it takes on the shape of a glass, and when in the solid state, it remains with its definite shape.
- Gaseous: water in a gaseous state can be observed in the atmosphere. In this case we find that their molecules are far apart from each other, having neither shape nor volume defined.
Changes in the physical state of water
When water changes from one physical state to another, we say that a change of state has occurred. This change occurs due to variations in temperature or pressure at specific values. Water, for example, changes from a solid to a liquid at 0 ºC at a pressure of 1 atm.

Let's look at the changes of state that occur in water below:
- Fusion: passage of a substance from solid to liquid. For this to occur, it is necessary for the substance to gain heat. Melting can be seen when we see ice melting.
- Vaporization: passage of a substance from a liquid to a gaseous state. For this to occur, the substance must gain heat. Vaporization can occur in three ways: evaporation, boiling and heating. The first is a slower vaporization. An example is when we put clothes on the clothesline to dry. The second, in turn, is faster than the previous one, with the formation of bubbles being observed. It can be checked when we put water to boil. The third occurs much faster than the other forms. When a drop of water falls on a hot plate, for example, we can observe the heating.
- Solidification: passage of a substance from a liquid to a solid state. For this to occur, the substance must lose heat. When liquid water forms ice in the freezer, for example, we are observing solidification.
- Condensation: also called liquefaction, occurs when a substance changes from a gaseous state to a liquid, losing heat. Condensation is responsible for the formation of clouds.
- Sublimation: happens when a substance goes directly from a solid to a gaseous state. The reverse process is called resublimation or it can also be called sublimation.
To learn more about this important process for the environment and humans, read: water cycle.
Let's review what we learned?
The following figure presents letters and numbers that indicate, respectively, the physical states of the water and its changes. Identify the status indicated by each letter and the changes indicated by each number.
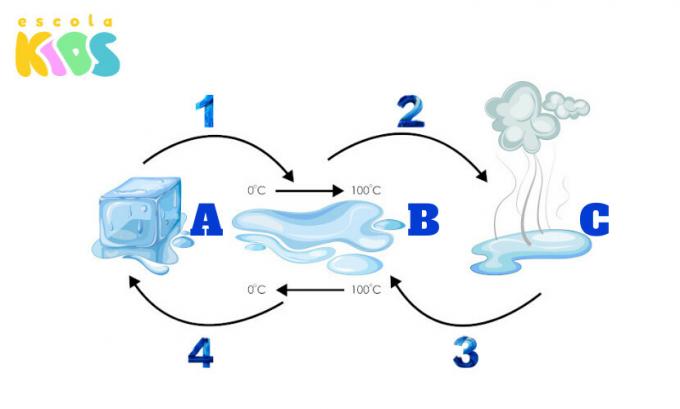
Did you identify? Check out the answer below:
A- Solid
B- Net
C- Gaseous
1- Fusion
2- Vaporization
3- Condensation
4-Solidification



