Antoine Laurent of Lavoisier he was born on August 26, 1743, in Paris.His father was a wealthy lawyer and his mother died early in his life. His father and aunt sent him to Colégio Mazzarino to study law.However, he had a greater interest in the area of Science.
At the age of 22, he has already shown his great ingenuity by designing a lighting project for the streets of Paris, for which he won a gold medal from the Academy of Sciences.At age 25, he was elected a member of the prestigious Royal Academy of Sciences of France.
At the same age he bought shares in Ferme Générale, associating himself with that private institution that collected taxes from the people in the name of the French crown. His aim was to cover the costs of his experiments and research.
At the age of 26, he met Marie Anne Pierrette Paulze (1758-1836), who was the daughter of one of Ferme Générale's major partners. Lavoisier and Marie Anne were married when she was just 13 years old, and he was 29 years old. But this marriage turned into a great union between them, as Marie Anne helped Lavoisier in his research, being his partner and assistant.
She helped him by assembling the apparatus for his experiments, as well as translating scientific and philosophical works.

Lavoisier and his wife and assistant, Marie Anne
Lavoisier was one of the great scientists of history, being considered by many as the father of modern chemistry. He made detailed observations and, unlike most, carefully planned his experiments, measuring the mass of materials before and after the chemical transformations.
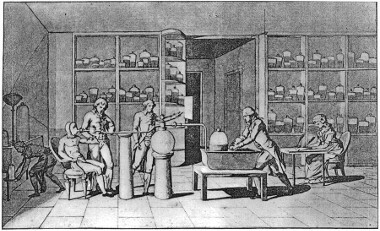
Illustration of Lavoisier's laboratory where he was experimenting with the help of his wife (who is sitting to the right, taking notes)
Among his main discoveries, we can highlight:
* Discovery of oxygen and the relationship between respiration and combustion reaction:In 1774 Priestley had discovered a new gas, which Lavoisier began to study and experiment with. With the data obtained, he demonstrated that that new gas was necessary for combustion to take place, that is, without its presence, there was no burning.
Lavoisier called this gas oxygen, word that comes from greek oxy, which means "acid", and gen, “generator or producer”. He named it because his experiments led him to conclude that this new gas was present in all acids, which later turned out to be a wrong conclusion, but still the name remained.
To see how true this discovery by Lavoisier is, light a candle and then cover it with a glass vial. In time, you will see that the candle will go out because all the oxygen inside the bottle will be consumed in burning the candle and will run out.
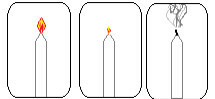
Candle experiment to confirm Lavoisier's discovery
* Composition of the air: Lavoisier showed that this same gas (oxygen) was part of atmospheric air and was the gas we breathe. He also showed that atmospheric air was a mixture of oxygen with another gas that did not participate in the combustion reaction. Today we know that this other gas is nitrogen.
These discoveries by Lavoisier represented a revolution for the ideas of the time about chemical transformations and debunked the theory of phlogiston, which was a theory that every flammable (burning) substance contained the phlogiston — a mysterious fluid lost in the moment. of combustion.
* Mass conservation law or Lavoisier's law: Lavoisier showed that, in combustion reactions, the masses of all substances involved remained unchanged, that is, the mass that he had weighed at the beginning, before the reaction, was the same as at the end of the reaction, as long as the reaction was done in a container closed. With that, he reached the famous Law of Conservation of Mass, which says that, in a chemical reaction, the mass of the reactants is equal to the mass of the products.
Today this law is best known by the following statement:
"In nature, nothing is created, nothing is lost, everything is transformed."
* Water composition: Lavoisier showed, in 1783, that water was the result of the combination of two parts of hydrogen and one of oxygen (H2O);
* Animal metabolism: Antoine Lavoisier showed that the metabolism of animals was an internal combustion, in which carbon and the hydrogen that was obtained from food reacted with oxygen and produced carbon dioxide and Water.
* Modern nomenclature for elements: In 1789, Lavoisier released the work entitled Elementary Chemistry Treaty, in which he provided a modern nomenclature for 33 elements, which, according to today's correct concept, are actually substances. This was important because alchemy used to use obscure language to refer to the elements.
Unfortunately, Lavoisier came to a tragic end. In that same year of 1789 the French Revolution took place, which overthrew the existing political order. The people rebelled against the excesses of the crown, and members of the Ferme Générale were considered enemies, among them Lavoisier.
He was arrested in November 1793 and, on May 8, 1794, was guillotined in the middle of Place de la Révolution in Paris, now Place de la Concorde.

Egyptian obelisk at the revolutionary guillotine site in the center of Place de la Concorde on June 9, 2014 in Paris, France *
* Image with editorial credit: Veniamin Kraskov / Shutterstock.com
By Jennifer Fogaça
Graduated in Chemistry