Covalent bond is a type of interaction between atoms that have high electronegativity, that is, high tendency to receive electrons. The chemical elements commonly involved in this type of bond are:
Hydrogen (H)
Beryllium (Be)
Boron (B)
Carbon (C)
Nitrogen (N)
Phosphorus (P)
Oxygen (O)
Sulfur (S)
Fluorine (F)
Chlorine (Cl)
Bromine (Br)
Iodine (I)
a) Nature of the elements involved
The chemical elements that have high electronegativity and, consequently, carry out covalent bonds are:
Hydrogen
Ametals
b) Occurrence of covalent bond
Depending on the nature of the chemical elements involved in the covalent bond, it can occur as follows:
Between two hydrogen atoms;
Between a non-metal atom and hydrogen;
Between atoms of the same chemical element (nonmetal);
Between atoms of different chemical elements (both non-metals).
c) Number of electrons each atom must receive
The number of electrons that each nonmetal or hydrogen atom receives in a bond is related to the octet rule.
According to the octet rule, an atom is stable when it acquires eight or two electrons (only in the case of Hydrogen) in the valence shell. If an atom has five electrons in its valence shell, for example, it must receive three electrons to achieve stability.
NOTE: Beryllium and Boron are exceptions to the octet rule, as they become stable, respectively, with 4 and 6 electrons in the valence shell.
The number of electrons in the valence shell can be easily determined by analyzing the chemical element family. In the table below, we have the number of electrons in the valence shell referring to the family to which the element belongs and the number of electrons it needs to receive to achieve stability:

d) Covalent bond principle
As in the covalent bond all involved atoms have a tendency to receive electrons, obligatorily, there will be between them a sharing of the electrons present in the valence shell (the level furthest from the nucleus).
Sharing occurs when an electron from the valence shell of an atom becomes part of the same electronic cloud that surrounds another electron from the valence shell of another atom.
Each hydrogen atom, for example, has an electron in the valence shell. When two electrons become part of the same cloud, each Hydrogen starts to have two valence electrons, that is, it stabilizes.
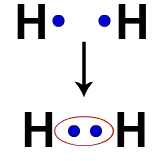
Electrons of two hydrogen atoms occupying the same electron cloud
e) Formulas used in covalent bonding
1ª) molecular formula
It is the indication of the number of atoms of each element that form the molecule originated from covalent bonds.
Example: H2O
In the water molecule, we have 2 hydrogen atoms and 1 oxygen atom.
2ª) structural formula
The structural formula is the demonstration of the organization of the molecule, that is, it demonstrates the bonds between atoms. For this, dashes that represent the bond of each atom are used:
Simple (?): Indicates that the atom shared only one electron from its valence shell with another atom and vice versa;
Double (?): Indicates that the atom shared two electrons from its valence shell with the other atom and vice versa;
Triple (≡): Indicates that the atom has shared three electrons from its valence shell with another atom and vice versa.
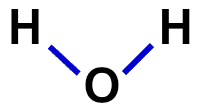
Structural formula of water
3ª) Lewis' electronic formula
The Lewis electronic formula also represents the organization of the molecule (electronic formula), but it aims to demonstrate the sharing of electrons in atoms.
To build it, it is enough to respect the organization proposed in a structural formula and replace each trace of the bonds (single, double or triple) by “two balls”, which represent the electrons.
In the structural formula of water, for example, we have two simple bonds between Hydrogens and Oxygen. Thus, between them, we will have only two balls, delimited by an ellipse (which represents the electronic cloud).
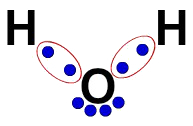
Lewis' electronic formula of water
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-ligacao-covalente.htm
