Carbon is a chemical element with an atomic number (Z) equal to 6, which means that the atoms that form it have six protons in their nucleus. Its molar mass is 12,011 g/mol and three isotopes of carbon are found in nature, which are: o carbon-12, carbon-13 and carbon-14. C-12 has six protons and six neutrons in the nucleus and is the most abundant.
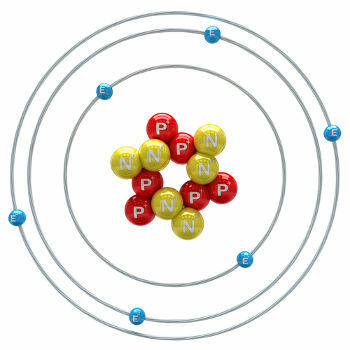
carbon atom-12 illustration
C-13 has seven neutrons and is the least abundant (1.01 to 1.14%). The C-14 has eight neutrons and is a radioactive element which emits β particles (electrons), being formed in the Earth's stratosphere when cosmic ray neutrons bombard the Nitrogen-14 present in these upper layers of the atmosphere. It is incorporated by all plants and animals and, knowing that its half-life is about 5730 years, is used to determine the age of fossils between 100 and 40,000 years. More details about C-14 and the dating technique can be seen in the text. What is Carbon-14?
Carbon is tetravalent, that is, it needs four more protons in its valence layer (outermost layer) to obey the octet rule. Therefore, it usually makes four covalent bonds, sharing four pairs of electrons with other elements as well as other carbons. These bonds can be single, double or triple and result in the formation of millions of different compounds. For this reason, an area of Chemistry was created, the
Organic chemistry, which studies the main compounds derived from carbon, with the exception of some cases that are of mineral origin, such as carbon dioxide (CO2), O carbon monoxide (CO), O calcium carbonate (CaCO3), sodium hydrogen carbonate or sodium bicarbonate (NaHCO3), between others. These compounds are studied in Inorganic chemistry.Carbon performs allotropy, forming simple substances, that is, substances that are formed only by bonds between carbon atoms. There are at least seven allotropes of carbon, which are graphite (alpha and beta), diamond, lonsdaleite (hexagonal diamond), chaoite, carbon (VI) and fullerenes. There are actually several types of fullerenes, which are synthetic allotropic forms of carbon. They have a polyhedral structure with a carbon atom at each vertex and an example is the Ç60 called buckminsterfullerene, and its structure looks like a soccer ball.
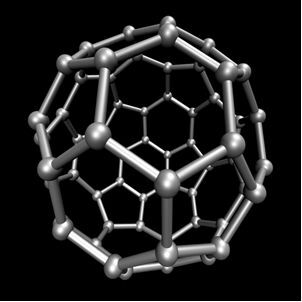
Carbon-60 (buckminsterfullerene)
However, among these allotropes of carbon, there are only two that are natural. graphiteIt's from Diamond. They differ only by the crystalline arrangement of atoms in space, as shown in the figure below, and this results in totally different physicochemical properties. Read the text carbon allotropy for more information.

The two natural allotropic forms of carbon are graphite and diamond.
Another synthetic allotropic form of carbon is the nanotubes (image below) that have broad biological applications, including medical diagnostics and treatments.
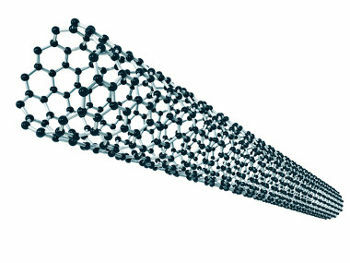
Illustration of a microscopic carbon nanotube
Thus, carbon is present in everything around us and within us, because he composes natural organic compounds — such as fossil fuels, which include oil, coal and natural gas, and other fuels such as ethanol and biofuels — agricultural products, among others. shape too synthetic organic compounds, such as synthetic fibers that make up fabrics, medicines, polymers that make up plastics and rubbers, insecticides, dyes and much more. Within us, animals and vegetables, carbon forms very important compounds, such as carbohydrates, such as sugar, glucose and cellulose; the proteins that form, for example, DNA, and together with lipids form the membranes of red blood cells and white blood cells.
All of this shows the importance of carbon for sustaining life. But it has also been associated with negative aspects, such as the intensification of greenhouse effect and the consequent global warming, this is because the main villain of these problems is its carbon dioxide compound (CO2). Mainly due to the large burning of fossil fuels that release this gas, the concentration of CO2 in the atmosphere has increased. As a greenhouse gas, it causes the problems mentioned. On the other hand, carbon dioxide is also present in vital reactions such as photosynthesis and respiration.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-carbono.htm

