Hydrocarbons, also called hydrogen carbides, are organic compounds whose composition has only atoms of carbon (C) and from hydrogen (H), thus having the general formula CxHy.
A hydrocarbon consists of a carbon structure to which hydrogen atoms bond in covalent bond.
It is the most important compound in organic chemistry.
All types of hydrocarbons oxidize easily, thus releasing heat. Most of them are not water-soluble.
Natural hydrocarbons are chemical compounds formed inside the Earth (more than 150 km from depth) at high pressure and reach zones of lower pressure through geological processes.
Where are hydrocarbons found?
The main source of hydrocarbon is oil. Because of this, the hydrocarbon is present in several derivatives such as kerosene, paraffin, natural gas, Gasoline, Vaseline, diesel oil, LPG (Liquefied petroleum gas), polymers (such as plastic and rubber), among others.
This organic compound constitutes 48% of Brazil's energy matrix.
The carbon chain that constitutes part of the composition of a hydrocarbon is tetravalent, that is, it can make four connections.
Carbon is able to bond with other carbon atoms and with hydrogens through simple links, doubles or triples.
Classification of hydrocarbons
The classification of hydrocarbons is based on three specificities: a form of the main carbon chain, the Connections of the carbon chains, the presence of alkyl radicals in the carbon chain and presence of heteroatoms dividing the carbon chain.
know more about hydrogen.
Main carbon chain shape
With regard to the shape of the main carbon chain, the classification of hydrocarbons is subdivided into aliphatic and cyclic.
Check out what each of these forms of carbon chain consists of.
aliphatic hydrocarbons
Aliphatic hydrocarbons are formed by carbon chains open or acyclic. In these chains, carbons are terminals.
Examples:
alkane
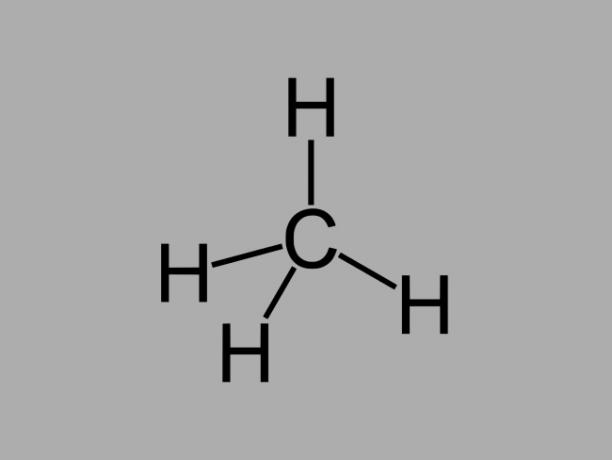
Alkane hydrocarbons, also called paraffins or paraffinic, are oily compounds where there are only single bonds between carbons.
The general formula for an alkane is CnoH2no + 2 (n = any whole number).
alkene
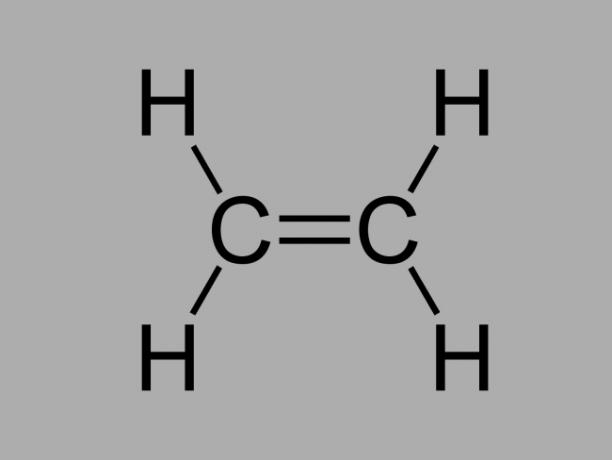
Also called olefin, alkene or ethylene hydrocarbon, alkene is a poorly reactive compound where there is a double bond between the carbons.
The general formula for an alkene is CnoH2no.
alkyne
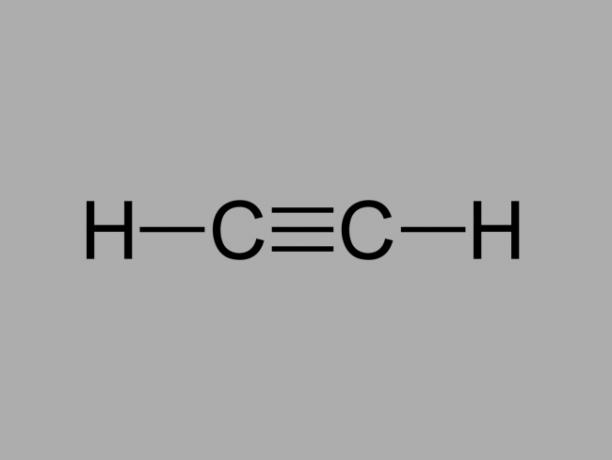
Also called methylacetylene, alkyne is a hydrocarbon where the existing bonds between carbons are triple.
The general formula for an alkyne is CnoH2no-2.
alkadiene

Also called dienes or diolefins, alkadienes are hydrocarbons where the bonds between the carbons are double.
The general formula for an alkadiene is CnoH2no-2.
Cyclic hydrocarbons
Cyclic hydrocarbons are formed by closed or cyclic carbon chains. These chains do not have terminal carbons.
Examples:
Cyclan
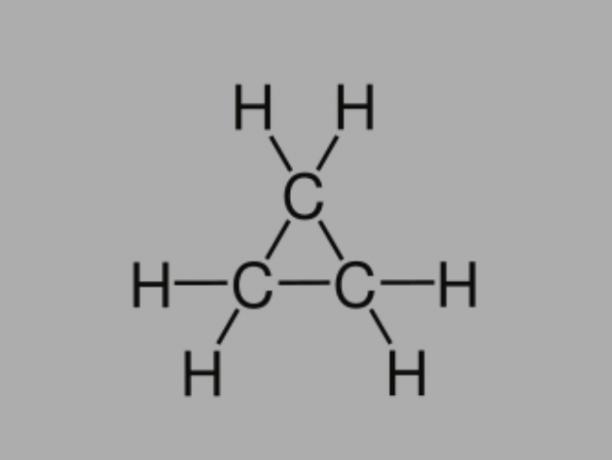
Also called cycloalkane, cycloparaffin or naphthenic hydrocarbon, cyclane is a saturated hydrocarbon, composed of single bonds.
It has a closed carbon chain and its general formula is CnoH2no.
Cyclonic

Also called cycloalkenes, cyclenes are unsaturated hydrocarbons, composed of double bonds.
A cyclene has a closed carbon chain and its general formula is CnoH2no−2.
cyclist
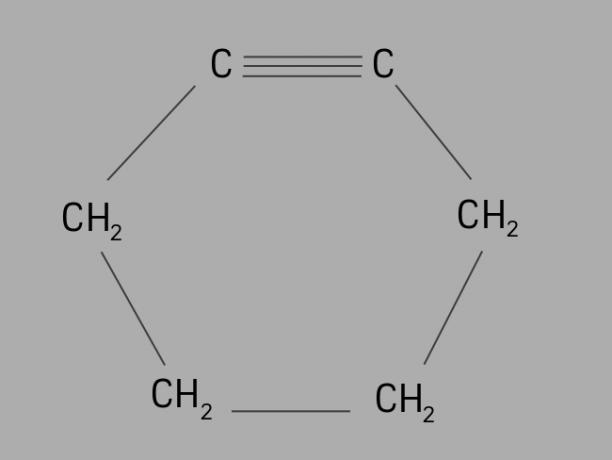
Also called cycloalkyne or cycloalkyne, cycline is a cyclic and unsaturated hydrocarbon.
It is formed by a closed carbon chain, with triple bonds and its general formula is CnoH2no-4.
Aromatic

or

Also called arenas, aromatic hydrocarbons are unsaturated compounds formed by double bonds.
An aromatic has a closed or cyclic carbon chain and its general formula is C6H6.
Type of bonding of carbon chains
Depending on the type of bonding of the carbon chains, hydrocarbons can be classified into saturated or unsaturated.
See below what each of these classifications consists of.
Saturated hydrocarbons
Saturated hydrocarbons are formed by simple links.
Examples: alkanes, cyclans.
unsaturated hydrocarbons
Unsaturated hydrocarbons are formed by double bonds or triples.
Examples: alkenes, alkynes, alkadienes.
Presence of alkyl radicals
Regarding the presence of alkyl radicals, hydrocarbons can have a carbon chain normal or branched.
normal carbon chain
A normal carbon chain hydrocarbon has no alkyl radicals.
Example: pentane
branched carbon chain
When a hydrocarbon has a branched carbon chain, it means that its main carbon chain has alkyl radicals.
Example: methylpropane
Presence of heteroatoms dividing the carbon chain
The sequential carbon chain may or may not be split depending on the presence of heteroatoms.
Homogeneous carbon chain
When a hydrocarbon has a homogeneous main carbon chain, it means that this chain is not divided by heteroatoms.
Heterogeneous carbon chain
If a hydrocarbon has a heterogeneous main carbon chain, this chain had its split carbon chain by a heteroatom.
Nomenclature of hydrocarbons
The nomenclature of hydrocarbons is defined through a combination of three parts:

The prefix identifies the quantity of carbons, the intermediate identifies the type of bond, and the suffix indicates the function to which the compound belongs (in this case, the class of hydrocarbons).
See below for the list of prefixes and intermediates that are combined to designate hydrocarbons.
Prefix list
| Number of carbons | Prefix |
|---|---|
| 1 | Met- |
| 2 | Et- |
| 3 | Prop- |
| 4 | But- |
| 5 | pent- |
| 6 | Hex- |
| 7 | Hept- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
Intermediaries list
| Connection Type | Intermediary |
|---|---|
| Just single calls | -an- |
| Pair | -en- |
| triple | -in- |
| two pairs | -dien- |
Look at some examples of hydrocarbon naming.
Examples:
CH3 – CH2 – CH2 – CH3
In the structural form above, we can see a 4-carbon compound that only has single bonds (indicated by the symbol “–”).
- Prefix for 4 carbons = but-
- Intermediate for single bindings= -an-
- Suffix of a hydrocarbon= -o
See that the union of prefix + intermediate + suffix gives rise to the name BUTANE.
CH2 = CH2
The structural form above has 2 carbons and 1 double bond (indicated by the “=” symbol).
- Prefix for 2 carbons = et-
- Intermediate for double bonds= -en-
- Suffix of a hydrocarbon= -o
See that the union of prefix + intermediate + suffix gives rise to the name ETHENE.
CH2 = CH - CH2 – CH3
CH3 – CH = CH2 – CH3
Note that both of the above structural forms have 4 carbons and 1 double bond (indicated by the “=” symbol).
Thus, we have:
- Prefix for 4 carbons = but-
- Intermediate for double bonds= -en-
- Suffix of a hydrocarbon= -o
See that the union of prefix + intermediate + suffix would give rise to the name BUTENE for the two structural forms.
However, note that the structural forms are not identical, so the nomenclatures cannot be either.
The difference between the two structural forms lies in the location of the double bond.
In this case, we must number the carbons in the chain from the end closest to the double. Therefore, in the cases in question, we must number from left to right.
In CH2 = CH - CH2 – CH3:
- CH2 will be the 1
- CH will be the 2
- CH2 will be the 3
- CH3 will be the 4
Note that the double bond is between the carbon 1 it's the carbon 2.
We must use the smallest number (1) to find the double bond: BUTENE -1
In CH3 – CH = CH2 – CH3:
- CH3 will be the 1
- CH will be the 2
- CH2 will be the 3
- CH3 will be the 4
Note that the double bond is between the carbon 2 it's the carbon 3.
We must use the smallest number (2) to find the double bond: BUTENE -2
According to IUPAC (International Union of Pure and Applied Chemistry – International Union of Pure and Applied Chemistry), the location should be indicated slightly before the being located (in the case of the structural formals above, the double bond, represented by the intermediate “-en-”).
With that, we have a second way, which by the way is the most correct, of writing the nomenclature of the available structural forms.
CH2 = CH - CH2 – CH3: BUTENE -1 or BUT-1-ENO (more correct form)
CH3 – CH = CH2 – CH3: BUTENE -2 or BUT-2-ENE (more correct form)
Learn more about IUPAC and nomenclature.

