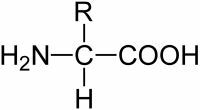
Ionizing is the action of separate or convert to ions. In the area of Chemistry, ion, ion or ion is an electrically charged particle, atom or molecule. It is any part of an atom or molecule displaced by the application of energy.
Ionizing is the process of transforming neutrally charged atoms into their respective ions.. The energy required for this transformation varies by element.
The formation of ions from atoms or molecules can occur in several ways. In certain chemical reactions, the ionizing process takes place by electron transfer.
Every atom is electrically neutral since it has the same number of protons and electrons. Likewise, substances formed by groups of atoms also have an electrical charge balance, being electrically neutral,
However, the moment an atom or group of atoms loses or gains electrons, it ceases to have neutrality and becomes ions. When losing electrons, the atom or group of atoms will have a positive charge and if it gains electrons, the charge will be negative.
For example, chlorine atoms react with sodium atoms to form sodium chloride, with electron transfer from sodium to chlorine, forming sodium ions (Na +) and chlorine ions (Cl -).
Learn more about ions.