valence layer it is the outermost layer (or level) (farthest from the nucleus) of an atom, that is, the one furthest from the nucleus. Therefore, it has the so-called outermost electrons or valence electrons.
The number of levels an atom can have varies from 1 to 7, which have the following sublevels (in yellow):
level K (1st layer): sublevel s
level L (2nd layer): sublevels s and P
level M (3rd layer): sublevels s, P and d
level N (4th layer): sublevels s, P, d and f
level O (5th tier): sublevels s, P, d and f
P level (6th tier): sublevels s, P and d
level Q (1st layer): sublevels s and P
Each of the sublevels holds a different number of electrons. Look:
sublevel s holds a maximum of 2 electrons;
sublevel p holds a maximum of 6 electrons;
sublevel d holds a maximum of 10 electrons;
sublevel f holds a maximum of 14 electrons.
Thus, if the valence shell of a certain atom is M, the maximum number of electrons that can be present in it are 18 (2 electrons from the s sublevel + 6 electrons from the p sublevel + 10 electrons from the sublevel d).
To determine the valence shell of an atom and how many electrons it has, there are two ways, namely:
→ Determination of the valence shell and its number of electrons from electronic distribution
Electronic distributions are always made through the Linus Pauling diagram, represented below:

Representation of a Linus Pauling diagram
Ordinary atomic number (which indicates the number of electrons in an atom), we do the electronic distribution. For example, an atom of atomic number 50:

Electronic distribution of the atom of atomic number equal to 50
Analyzing the above distribution, we have that the level furthest from the nucleus is the 5th (N level), in which we have the presence of 4 electrons (two in the s sublevel and 2 in the p sublevel).
→ Determination of the valence shell and its number of electrons from the Periodic Table
The table is arranged in periods (horizontal columns), which indicate the number of levels of an atom, and groups or families (vertical columns). The period is used to determine the valence layer, and the families are used to determine the number of electrons.
a) Knowing the period of the chemical element
The Periodic Table presents a total of seven periods, which number is related to the number of levels present in the Linus Pauling diagram. So, if we know the period in which the chemical element it is found in the table, automatically, we know how many levels your atoms have, the valence layer being the level farthest from the nucleus.
1st Example: Chemical element Potassium
Potassium is positioned in the fourth period of the Periodic Table, so its atom has four levels, the fourth level being the valence layer, which is confirmed through its distribution electronics.
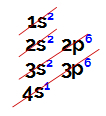
Electronic distribution of element potassium
2nd Example: Chemical element Fluorine
Fluorine is positioned in the second period of the Periodic Table, so its atom has two levels, the second level being the valence layer, which is confirmed through its distribution electronics.

Electronic distribution of the fluorine element
3rd Example: Indium chemical element
Do not stop now... There's more after the advertising ;)
The Indian is positioned in the fifth period of the Periodic Table, so his atom has five levels, the fifth level being the valence layer, which is confirmed through its distribution electronics.

Electronic distribution of the Indium element
b) Knowing the family or group of the chemical element
Knowing the family or group in which the element is positioned, we also know the number of electrons present in that element's valence shell.
Elements of family A
The elements of families A are positioned in columns 1, 2, 13 to 18 of the Periodic Table. Each of these columns receives a number (1 to 8, Roman numeral), which indicates exactly the number of electrons in the valence shell of these elements:
Column 1 - IA family = all have 1 electron in the valence shell;
Column 2 - family IIA = all have 2 electrons in the valence shell;
Column 3 - family IIIA = all have 3 electrons in the valence shell;
Column 4 - IVA family = all have 4 electrons in the valence shell;
Column 5 - VA family = all have 5 electrons in the valence shell;
Column 6 - VIA family = all have 6 electrons in the valence shell;
Column 7 - family VIIA = all have 7 electrons in the valence shell;
Column 8 - family VIIIA = all have 8 electrons in the valence shell.
See some examples of the determination of the number of valence electrons of some elements of family A:
Example 1: chemical element barium
Barium is located in the IIA family, so it has two electrons in the valence shell, which is confirmed by its electronic distribution:
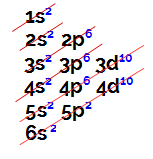
Electronic distribution of the Barium element
Example 2: Antimony chemical element
Antimony is located in the VA family, so it has five electrons in the valence shell, which is confirmed by its electronic distribution:

Electronic distribution of the antimony element
Example 3: Xenon chemical element
Xenon is located in the VIIIA family, so it has eight electrons in the valence shell, which is confirmed by its electronic distribution.

Electronic distribution of the Xenon element
NOTE: the only chemical element belonging to the A family that does not comply with the proposed rule is Helium. It belongs to the VIIIA family, but has only two electrons in the valence shell. This is because its atomic number is 2, so it is impossible for it to have 8 electrons in the valence shell like the other elements in the family.
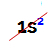
Electronic distribution of a helium atom
Family B elements
The elements of families B are positioned in columns 3 to 12 of the Periodic Table. As well as the A families, there are also eight B families, which are represented by Roman numerals. Unlike the A families, the number of the B family does not determine the number of electrons in the valence shell.
The number of electrons in the valence shell of an element of family B is always equal to 2, regardless of the atomic number and position in the table. The electronic distributions of californium (98Cf) and hassius (108hs) prove this:
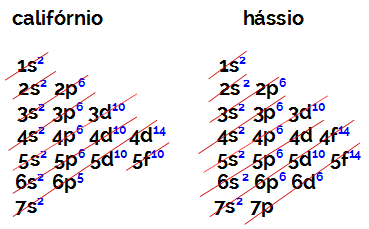
The electronic distribution of californium has as the most energetic sublevel the 5f10, and the o of hassius is 6d6. In both cases, the furthest sublevel from the nucleus is the seventh level, and both have two distributed electrons.
By Me. Diogo Lopes Dias

