You Hydrocarbons are composed formed by carbon (C) and hydrogen (H). The flexibility of carbon, which is the main element of these compounds, favors the existence of a huge diversity of structures Therefore, some properties, such as melting point and boiling point, may differ between one hydrocarbon and another.
They are mostly molecules apolar, with strengths intermolecular induced dipole type and density less than The from water. The identification of these compounds can be done through the nomenclature, which follows the rules established by the International Union of Pure and Applied Chemistry (Iupac).
Read too: Carbon - one of the most abundant elements in the Universe
Properties of hydrocarbons

Polarity: hydrocarbons without the presence of heteroatoms are apolar.
Intermolecular Forces: the bonds between the molecules of a hydrocarbon are of the type induced dipole.
melting point and andboiling: they vary according to the size, function and structural organization of the molecule.
physical state: under normal conditions of temperature and pressure, hydrocarbons with four or fewer carbon atoms are in a gaseous state. Those with 5 to 17 carbons are in a liquid state, and hydrocarbons with more than 17 carbons are solid substances.
Density: is smaller than the density of water, ie, less than 1.0 g/cm³.
Reactivity: aliphatic and unsaturated hydrocarbons are poorly reactive; unsaturated compounds are more likely to react with other molecules, and cyclic hydrocarbons with up to five carbons are very reactive.
Do not stop now... There's more after the advertising ;)
Classification of hydrocarbons
The hydrocarbons can be classified by the structural organization of the chain and by the establishments.Unsaturation is the presence of double(s) or triple bond between carbons, is the occurrence of pi type connections (π). already the branches they are like “branches” attached to a larger hydrocarbon structure. Cyclic chains can also contain branches and/or unsaturations – the structural organization of closed-chain hydrocarbons forms polygons such as square, triangle, hexane, among others.
Regarding the arrangement of atoms, a hydrocarbon can have a normal or branched chain.
→ C hydrocarbonnormal, linear or straight line: the one with a string that has only two ends.
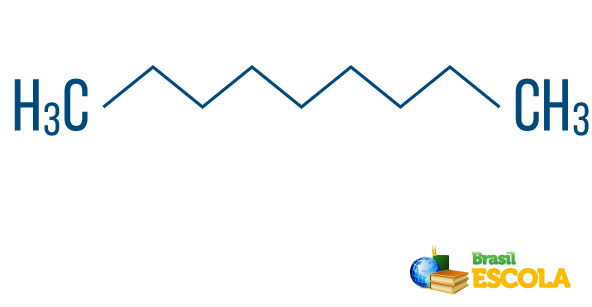
→ C hydrocarbonhates branched: the one with a chain that has more than two ends. In order to know where and what the branches are, it is important to select the main chain correctly. The main chain must contain all unsaturations and heteroatoms (if any), as well as the greatest number of sequential carbons. Carbons that are not contained in the main chain are branches.
Example:

With respect to its "closure", a hydrocarbon can have a closed, open or mixed chain.
→ Chain hydrocarbonss closed or cyclic: those with chains in which the atoms organize themselves forming a cycle, a polygon or an aromatic ring (closed hydrocarbon with alternating unsaturations). Do not have loose ends unless there is a branch. Each vertex of the polygon represents a carbon and its respective hydrogen bonding agents.
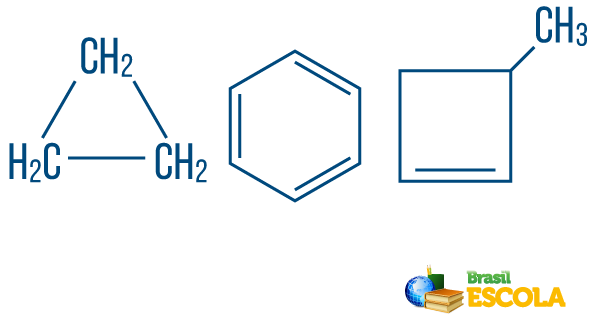
→ Open chain or acyclic hydrocarbons: are those with chains that have at least two ends.

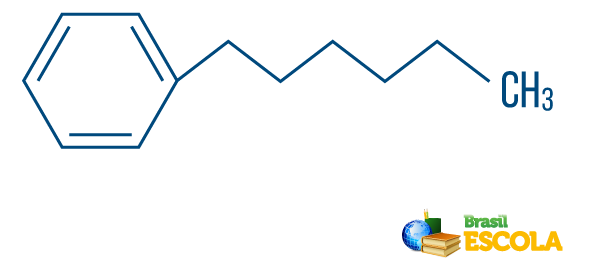
→ Hydrocarbon of mixed type string: is formed by a ring or cyclic chain attached to a linear part; it has at least one end.
Read too: Aromatic hydrocarbons - examples and properties
Nomenclature of hydrocarbons
For each type of hydrocarbon, there is a naming rule established by Iupac. The nomenclature of these compounds is done as follows:
1st part: location and name of branch(s) (if any);
2nd part: the term applies cycle if the compound is a closed chain, but if it is an aliphatic (open) chain, it will not be necessary;
3rd part: prefix indicating how many carbons there are in the main chain;
4th part: location and infix indicative of the type of unsaturation in the molecule;
5th part: suffix “o” proper to hydrocarbons.
If the molecule has an open structure, without branching, the nomenclature will start in part 3.
The following table shows the required information (prefix, infix and suffix) for hydrocarbon nomenclature in general. O prefix varies with the number of carbons; O infix, according to the number of unsaturations; it's the suffix“O" refers to compounds of the hydrocarbon type.
Prefix |
Infix |
Suffix |
||
1 carbon |
met- |
Single calls only |
-an- |
-O |
2 carbons |
et- |
|||
3 carbons |
prop- |
1 double bond |
-en- |
|
4 carbons |
but- |
|||
5 carbons |
pent- |
2 double bonds |
-dien- |
|
6 carbons |
hex- |
|||
7 carbons |
hept- |
1 triple bond |
-in- |
|
8 carbons |
oct- |
|||
9 carbons |
non- |
2 triple links |
-diin- |
|
10 carbons |
dec- |
The first step in discovering the nomenclature of an organic compound is identify the main chain of carbons, which must contain the unsaturations and as many sequential carbons as possible. After identifying the main chain, the carbons must be enumerated – starting the count from the side closest to the branches and unsaturations (if any). THE localization will be the number of the carbon where the branch or unsaturation is found. Sometimes there is only one possible location for a radical or double or triple bond, so it is not necessary to express the location of the linking carbon in the nomenclature.

THE nomenclature for branches will be given by the number of carbons in each, plus termination line or ll. When there is more than one branch, alphabetical order is used.
See the examples below:
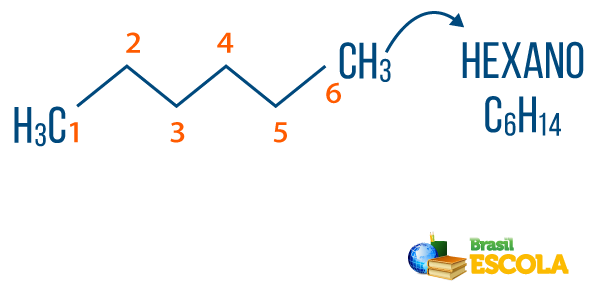
→ Example 1
CH3 – CH2 – CH3 → Propane
1st part: the prefix “prop-” indicates the chain has three carbons.
2nd part: the infix "-an-” signals that the molecule only makes connections of the type sigma or simple.
3rd part: the suffix "-O” is characteristic of hydrocarbons.
→ Example 2
CH2=CH-CH2-CH3 → But-1-ene
In hydrocarbons with unsaturation, it is necessary to number and locate the carbon in which the pair is found, and the numbering must be as small as possible. For this, the carbon count must start with the side closest to the double bond.
1st part: "but-” indicates that there are four carbons in the chain.
2nd part: "1-en" refers to unsaturation located between carbon 1 and 2.
3rd part: "-O" is the characteristic suffix of hydrocarbons.
→ Example 3

1st part: "3-ethyl" signals that there is a two-carbon branch on carbon 2.
2nd part: "-pent-" indicates the presence of five carbons in the main chain.
3rd part: "-an-" is the infix applied to unsaturated chains (no double or triple bonds).
4th part: "-O" is the characteristic suffix of hydrocarbons.
→ Example 4
For chains with more than one branch, place the radicals in the nomenclature in alphabetical order. If there are branches and unsaturations in the same molecule, the count of carbons in the main chain should be done in such a way that the sum of the location numerals is as small as possible.
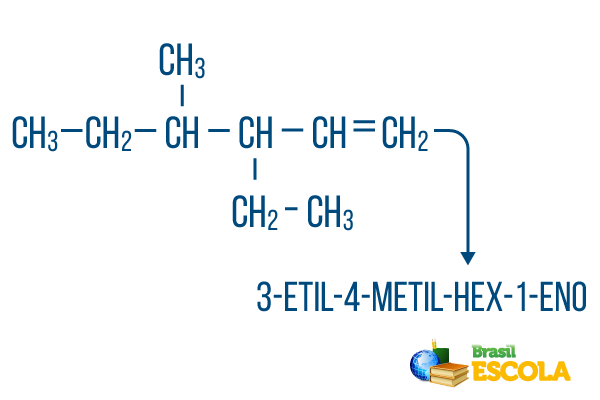
The main chain carbon count was done from left to right, and the sum of the unsaturation and branch location numerals is: 1+4+3 = 8. If the carbon count had been from right to left, the compound nomenclature would be 4-ethyl-3-methyl-5-ene, whose sum of locations would be: 4+3+5 = 12, which is greater than the other hypothesis, so it should not be used.
1st part: 3-ethyl-4-methyl makes reference to the radicals in alphabetical order and their respective locations.
2nd part: hex- means there are 6 carbons in the main chain.
3rd part: 1-en indicates the presence of a double bond on carbon 1.
4th part: "-O" is the characteristic suffix of hydrocarbons.
→ Example 5
For closed strings, the naming rules hold, but the word cycle starts the name of the compound, indicating that it is a closed or cyclic hydrocarbon.
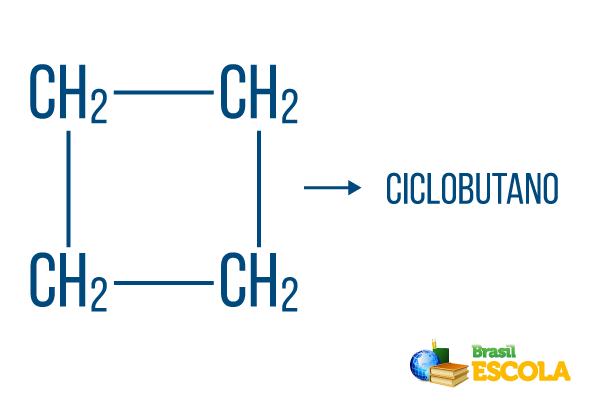
1st part: cycle- indicates that it is a closed string.
2nd part: -but- denotes the existence of 4 carbons in the chain
3rd part: -Ois the characteristic suffix of hydrocarbons.
Read too: Classification of carbon chains
Types of hydrocarbons
Hydrocarbons can be divided into alkanes, alkenes, alkynes and alkadienes - which are classified as according to the establishment of the chain (double(s) or triple bonds) - and cyclans, which are the chains closed.
→ Alkanes: are hydrocarbons that do not have unsaturation. The general formula for alkanes is CnoH2n+2, and the noomenclature it's composed by prefix + an + O.
You alkanes can be found in nature, like gas methane (CH4), which is released by animals and produced in decomposition processes, as well as in refineries and petrochemical industries. Compounds like propane (Ç3H7), butane (Ç4H10), which makes up our cooking gas (LPG), and the octane (Ç8H18), present in automotive fuel, are by-products of Petroleum.

→ alkenes or alkenes: are carbon chains that have an unsaturation, a double bond. Its general formula is CnoH2n, and yours noomenclature it's composed by Prefix + en + o.

O ethylene gas (Ç2H4), used in agriculture to accelerate the ripening of fruits, belongs to the function alkene. The compound is also used in the production of raw material polyethylene, used in the manufacture of plastic utensils.
→ Alkynes or acetylenic: hydrocarbons with a triple bond. Its general formula is CnoH2n - 2. THE nomenclature it's composed by prefix + in + o.

O acetylene or etine (C2H2) is a gas of the alkyne function used in welds and metal cuts. This compound can reach temperatures of up to 3,000 °C, which allows repairs to be carried out on submerged parts of a ship.

→ alkadienes or dienes: carbon chains with two unsaturations, that is, two double bonds between carbons. The general formula for this function is C.noH2n - 2. Realize that it's the same formula as alkynes, which means it can happen isomerism between compounds (same molecular formula for different compounds).
The nomenclature of an alkadiene is composed of Prefix + dien + o.
Example:

→ Closed chain hydrocarbons: the molecules organize themselves in a cyclic way, tend to form a polygon and, as in open chains, there may be establishments and/or ramifications. Cyclones, cyclenes, cyclines and benzenes are closed chain hydrocarbons.
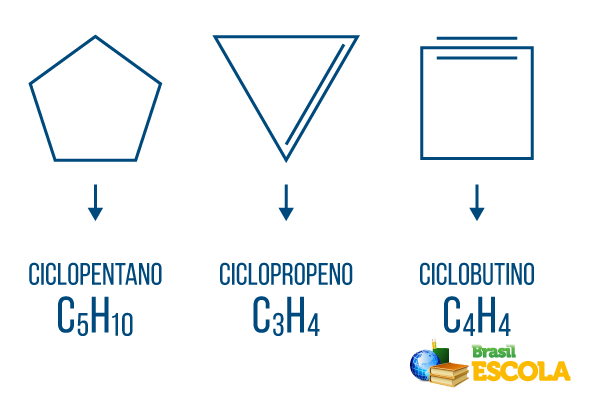
Cyclones or cycloalkanes: cyclic chains consisting only of single bonds. Its general formula is CnoH2n. Nomenclature: ciclo + prefix + an + o.
Cycles or cycloalkenes: closed hydrocarbon chains with an establishment. Its general formula is CnoH2n-2. Nomenclature: çiclo + prefix + en + O.
-
Cyclines or cycloalkynes: closed chain hydrocarbons with the presence of two double bonds. Its general formula is CnoH2n-4. Nomenclature: çiclo + prefix + in + o.
benzenes
Benzene is a type of closed-chain hydrocarbon with six carbons where the bonds vary between single and double. These compounds are toxic and highly carcinogenic, being used as organic solvents in chemical processes.
For the hydrocarbon to be considered aromatic, there must be at least one benzene ring, which is highly reactive, therefore subject to two or more replacements, which we'll see here as ramifications. When there are two linking radicals, we will have specific names for each pair of positions.
Radials on 1,2 carbons of benzene → ortho
Radicals at the 1,3 carbons of benzene → goal
Radials in carbon1,4 of benzene → for
Annomenclature of an aromatic compound is done as follows:
1st part: positioning of the ligands (ortho, goal or to).
2nd part: name of the radical or radicals attached to benzene (methyl, ethyl, propyl…). The name given to radicals follows the rule of other hydrocarbons.
- 3rd part: -Bpoison, which is the characteristic term of aromatic hydrocarbons.
Examples:
→ Ortho-dimethyl-benzene

1st part: Orto- indicates that the radicals are positioned on carbons 1 and 2.
2nd part: -dimethyl- refers to the two radicals, both with one carbon.
3rd part: -benzene is the characteristic term for aromatic hydrocarbons.
→ Ortho-ethyl-methyl-benzene
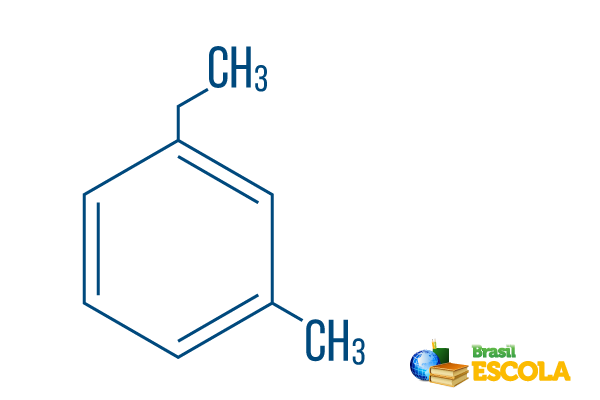
1st part: moh- indicates that the radicals are positioned on carbons 1 and 3.
2nd part: ethyl-methyl- refers to the amount of carbon in each radical, being ethyl The two-carbon branch and methyl branching with one carbon – placed in the nomenclature in alphabetical order.
3rd part: -benzene is the characteristic term for aromatic hydrocarbons.
→ para-diethyl-benzene
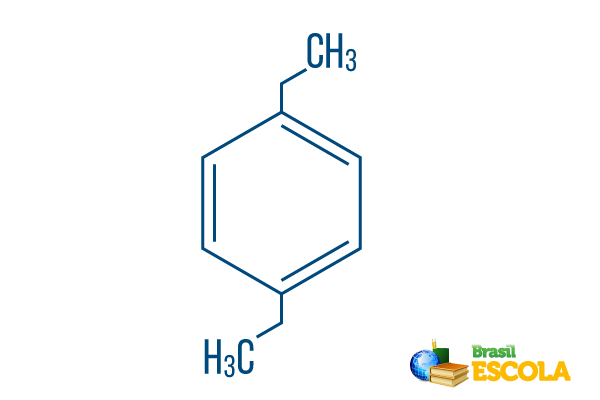
1st part: Pplow- indicates that the radicals are on carbons 1 and 4 of benzene.
2nd part: -diethyl- makes reference to two radicals of the type ethyl, that is, two branches with two carbons each.
3rd part: -benzene is the characteristic term for aromatic hydrocarbons.
Read too:Discovery of the structure of benzene
solved exercises
(Unesp) – Octane is one of the main constituents of gasoline, which is a mixture of hydrocarbons. The molecular formula of octane is:
a) C8H18
b) C8H16
c) C8H14
d) C12H24
e) C18H38
Answer: letter a). Analyzing the compound nomenclature octane, it is an alkane, that is, a molecule consisting only of single bonds. If the general formula for alkanes is CnoH2n+2, replacing “n” by eight, which is the amount of carbons in the main chain – and unique in this case –, we will have that the molecular formula of octane is C8H18.
(UFSCar-SP) – Consider the following statements about hydrocarbons.
I) Hydrocarbons are organic compounds consisting only of carbon and hydrogen.
II) Only straight-chain unsaturated hydrocarbons are called alkenes.
III) Cycloalkanes are saturated aliphatic hydrocarbons with the general formula CnH2n.
IV) Are aromatic hydrocarbons: bromobenzene, p-nitrotoluene and naphthalene.
The following statements are correct:
a) I and III, only.
b) I, III and IV only.
c) II and III only.
d) III and IV only.
e) I, II and IV only.
Answer: Letter a).
II – Alkenes are compounds with a double bond, that is, unsaturated, but they can have ramifications in their chain, not being exclusively linear.
IV - The bromethobenzene and p-nitrotoluene compounds belong to other organic functions.
by Laysa Bernardes
Chemistry teacher

