O It is madephotoelectric is a phenomenon of quantum origin that consists of issue in electrons by some material that is illuminated by electromagnetic radiation of specific frequencies. The electrons emitted by these materials are called photoelectrons.
Lookalso: quantum theory
Who discovered the photoelectric effect?
O It is madephotoelectric was discovered in 1886 by the German physicist Heinrichhertz (1857-1894). At the time, Hertz realized that the incidence of ultraviolet light on metal sheets helped the production of sparks. The theoretical explanation for the photoelectric effect, however, was only presented by the German physicist Albert Einstein, in 1905.
The doubt that existed at the time was related to the energykineticsFromelectrons that were ejected from the metal: this greatness nodependedgivesintensitygivesincident light. Einstein realized that the agent responsible for the ejection of each electron was a single photon, a particle of light that transferred part of its energy to electrons, ejecting it from the material, as long as its frequency was large enough to do so. To do so, Einstein used the ideas of the German physicist
MaxPlanck (1858-1947).Planck claimed that the light radiated by a black body it was quantized, that is, it had a minimum energy value, as in small packets. Einstein extended the idea to all electromagnetic waves and managed to solve the problem of the photoelectric effect. Einstein and Planck later received the award Nobel Prize in Physics for his discoveries related to the quantization of light.
Lookalso: What are photons?
How does the photoelectric effect work?
O It is madephotoelectric consists of the ejection of electrons from a material exposed to a certain frequency of electromagnetic radiation. The light packages, called photons, transfer energy to electrons. If this amount of energy is greater than the minimum energy needed to rip the electrons off, they will be ripped from the surface of the material, forming a chaininphotoelectrons.
Do not stop now... There's more after the advertising ;)
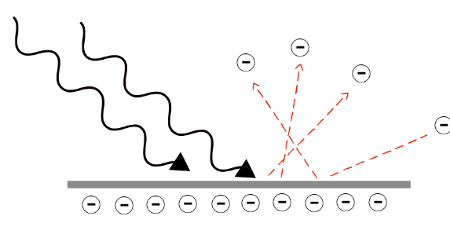
The energy of each photon It dependsinyourfrequency (f), therefore, there is a minimum frequency needed to strip the electrons from the material. The minimum energy that each photon must have to promote the photoelectric effect is called occupationwork. The following equation allows you to calculate the energy of a single photon of frequency f:
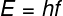
In the equation above, H is a physical constant called Planck's constant, with a value of 4.0.10-15 eV.s. The kinetic energy that the electron acquires after being hit by a photon is determined by the difference between the photon's energy and the work function (Φ):

The work function is a characteristic of each material and depends on how bound the electrons are in the material. Check a table with work function values, in units of eV (electron volts - each eV equals 1,6.10-19 J), for some metals:
Material |
Work function value (eV) |
Sodium |
2,28 |
Cobalt |
3,90 |
Aluminum |
4,08 |
Copper |
4,70 |
See too: Exercises on photoelectric effect
Technological applications of the photoelectric effect
The most famous technological application based on the photoelectric effect is the photovoltaic cell, used in solar panels to generate clean and renewable electricity.

By Rafael Hellerbrock
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
HELERBROCK, Rafael. "What is a photoelectric effect?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/fisica/o-que-e-efeito-fotoeletrico.htm. Accessed on June 27, 2021.
