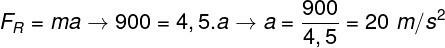We know that gaseous transformations can be:
isochoric
Transformation in which the gas volume remains constant.
isothermal
Transformation in which the temperature remains constant.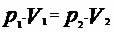
Isobaric
Transformation in which the pressure remains constant.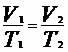
Now let's look at some applications of these equations in exercises:
1) (FUVEST - SP) A non-deformable, hermetically closed container contains 10 liters of a perfect gas at 30°C, supporting a pressure of 2 atmospheres. The gas temperature is increased until reaching 60°C.
a) Calculate the final gas pressure.
b) Sketch the pressure versus temperature graph of the described transformation.
Solution:
Letter a)
Considering that the gas volume is constant, the transformation is isochoric.
Thus,
Substituting the values provided by the problem in the isochoric transformation equation, we have:
Thus, we can conclude that pressure and temperature are directly proportional quantities.
Letter B)
From the resolution of the previous item, we can sketch the graph of pressure as a function of temperature (pressure x temperature).
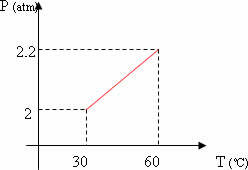
2) (FAAP - SP) At 27º C, an ideal gas occupies 500 cm3. What volume will it occupy at -73º C, the transformation being isobaric?
It is known that:
T1 = 27º C = 300 K
T2 = -73°C = 200K
V1 = 500 cm3
V2 = ?
From the isobaric transformation we have to: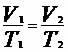 , thus:
, thus:
Do not stop now... There's more after the advertising ;)

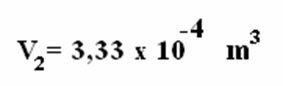
We can conclude that, for the isobaric transformation, volume and temperature are directly proportional.
3) (UNIMEP - SP) 15 liters of a given gaseous mass are at a pressure of 8.0 atm and a temperature of 30°C. When undergoing an isothermal expansion, its volume becomes 20 liters. What will the new gas pressure be?
From the statement we have:
V1 = 15 liters
V2 = 20 liters
P1 = 8.0 atm
P2 = ?
T = 30º C = 303 K (CONSTANT TEMPERATURE)
Using the isothermal transformation equation, we have: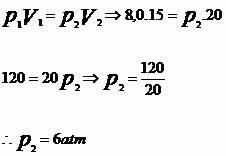
According to the isothermal transformation, pressure and volume, in a gaseous transformation, are inversely proportional quantities.
*Note: To solve problems involving gas transformations, we must ALWAYS use the temperature in the absolute scale (Kelvin).
By Kleber Cavalcante
Graduated in Physics
Brazil School Team
See more!!!
Gas Transformations
See how each of the transformations takes place.
Thermology - Physics - Brazil School
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Gas Transformations: Resolved Exercises."; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/transformacoes-gasosas-exercicios-resolvidos.htm. Accessed on June 27, 2021.