Isomerism it is a natural phenomenon in which different substances (in terms of chemical and physical properties) have exactly the same molecular formula, as in the following example:
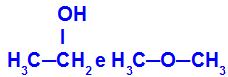
Different substances that have the same molecular formula
Ethanol (left) has the molecular formula C2H6O, which also occurs with methoxyethane (right), so they are isomers.
Types of Isomerism
→ flat isomer
It is the type of isomerism that studies the structural differences between isomers.
The) Occupation
It is the type of flat isomerism in which the difference between substances is based on the difference between the organic functions to which they belong. See some examples:
Propanone and propane:
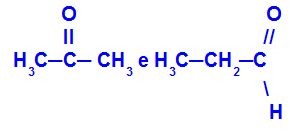
Isomers belonging to different functions
Propanone (left) belongs to the ketone group, and propanal (right) belongs to the aldehyde group.
B) Jail
It is the type of flat isomerism in which the difference between substances is based on the difference between the chains they present. See an example:
2-methyl-propane and butane
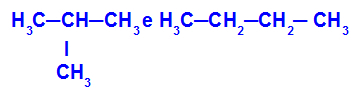
Isomers that have chains with different classifications
In the example, we can see that 2-methyl-propane (on the left) has a branched chain, and butane (on the right) has a normal chain.
ç) Position
It is the type of flat isomerism in which the difference between substances is based on the difference in position of a component present in their chains. See an example:
1-chloro-propane and 2-chloro-propane
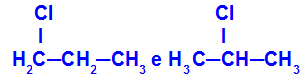
Isomers that have components at different positions in the chain
In the example, we can see that 1-chloro-propane (on the left) has the chlorine positioned on carbon 1, and 2-chloro-propane (on the right) has the chlorine positioned on carbon 2.
d) Metameria or compensation
NOTE: Isomer is valid exclusively for heterogeneous strings.
It is the type of flat isomerism in which the difference between the substances is based on the difference in the position of a heteroatom present in their chains. See an example:
Example: Methoxypropane and Ethoxyethane
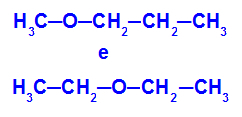
Isomers that have heteroatoms at different positions in the chain
In the example, we can see that, in methoxypropane (left), oxygen has a radical with one carbon on one side and three carbons on the other. In ethoxyethane (right), oxygen has a radical with two carbons on one side and two carbons on the other.
and)Tautomery
It is a particular case of plane function isomerism and occurs in only three organic functions:
Aldehyde
ketone
Enol
This type of isomerism works on the fact that there is a chemical balance between an enol and an aldehyde and between an enol and a ketone, that is, these components are constantly converting into each other. See an example:
Prop-2-en-1-ol and propanone
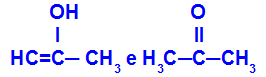
Isomers belonging to different functions
In the example, we can see that Prop-2-en-1-ol (on the left) belongs to the group of enols, and propanone (on the right) belongs to the group of ketones, so they are isomers of tautomery.
→ Space Isomerism
It is a type of isomerism defined by the spatial analysis of the molecule.
a) Geometric isomers
Do not stop now... There's more after the advertising ;)
It is a type of spatial isomerism that occurs when the substance has molecules with the following characteristics:
Closed chain that has two carbons with two different ligands, as in the example below:

Closed structure with geometric isomerism
Open chain that has a double bond between carbons and, in each of these carbons of the double bond, there are two different ligands, as in the example below:
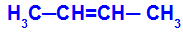
Open structure with geometric isomerism
Geometric isomerism is divided into two groups:
1O Group: cis-trans
It occurs when the two ligands of one carbon are strictly equal to the two ligands of the other carbon, whether in the open structure or in the closed structure.
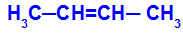
Chain of a substance that has cis-trans isomerism
The isomer will be called cis when the same ligands are in the same plane.

Example of a cis isomer
The isomer will be called trans when the different ligands are in the same plane.

Example of a trans isomer
2O Group: E-Z
It occurs when the two ligands of one carbon are different in relation to the two ligands of the other carbon, either in the open structure or in the closed structure.

Chain of a substance that has E-Z isomerism
The isomer will be called E when the ligands with the highest atomic number are in opposite planes. In the example below, the carbon on the left has the Br with the highest atomic number (35) and, in the other, there is oxygen (8).
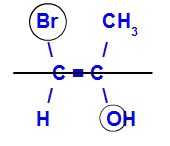
Example of an E-isomer
The isomer will be called Z when the ligands with the highest atomic number are in the same plane. In the example below, the carbon on the left has the Br with the highest atomic number (35) and, in the other, there is oxygen (8).

Example of a Z-isomer
B) optical isomer
It is a type of spatial isomerism that only occurs if the substance has molecules with chiral carbon (the one that has four different ligands) in their structures. The molecule with chiral carbon has the ability to polarize and deflect light, as follows:
To the right (right-hand isomer)
To the left (levorotatory isomer)
A compound with optical activity always has active isomers (called optical antipodes) and inactive isomers (mixture between two active isomers, a mixture called racemic).
We can use the following formula to determine the number of active (IOA) and inactive (IOI) isomers of a compound that has chiral carbon:
IOA = 2no
IOI = 2no
2
Below is an example of a compound that has geometric isomerism:
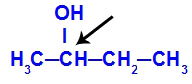
Chiral carbon featured in butan-2-ol
The structure of butan-2-ol has the following ligands:
Methyl (CH3)
Ethyl (CH3-CH2)
Hydrogen (H)
Hydroxyl (OH)
Because it has only one chiral carbon, therefore, butan-2-ol has:
Active isomers:
IOA = 21
IOA = 2
Inactive isomers:
IOI = 21
2
IOI = 2
2
IOI = 1
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is isomerism?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-isomeria.htm. Accessed on June 27, 2021.