
Its general formula is: ÇnoH2n.
The n represents any value of whole and positive numbers, that is, natural numbers (1, 2, 3,4...). For example, the cycloalkane below is cyclopentane, which has 5 carbons, so n = 5; thus, the required amount of hydrogens will be 2.n=2.5=10. Note how this happens by looking at the structure below:

Cycloalkanes have two more names, which are: cycloparaffins and naphthenic hydrocarbons, because they are found in paraffins and naphthenic-based oil.
Cycloalkanes that have 3 to 5 carbons in their chain are reasonably reactive; however, those with more than 5 carbons are quite stable.
| *Nomenclature of Unbranched Cycloalkanes: |
Its nomenclature is very similar to that of alkanes*, but with the addition of the word cycle at the beginning. See the example and observe the naming rule for these compounds below:


*Nomenclature of Branched Cycloalkanes:
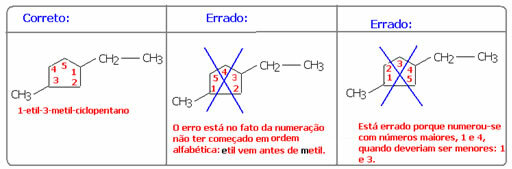
The only difference is that you have to quote the branch initially. If there is more than one branch, the chain is enumerated, remembering that it is necessary to start from the branch based on alphabetical order and so that the branches are cited with the smallest numbers possible. In the example below, this was done: notice that the numbering was 1 and 3, not 1 and 4. In addition, it started with ethyl and not methyl, as the example is in alphabetical order:
* To clarify any doubts about alkanes nomenclature, access the text "Alkanes nomenclature”
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team


