Hybridization is the name given to the fusion or union of incomplete atomic orbitals, a phenomenon that increases the number of covalent bonds that an atom can do. Remembering that orbital is the region of the atom where there is greater probability of finding an electron.
To know the number of bonds an atom makes and understand the phenomenon of hybridization, it is necessary to know some fundamental points about the atom:
1O point: the energy sublevels
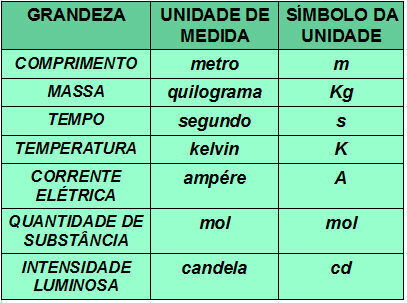
The energy sublevels that an atom can have are s, p, d, f.
2O point: number of orbitals per sublevel
Each energy sublevel has a different amount of orbitals, as we can see below:
Sublevel s: 1 orbital;
p sublevel: 3 orbitals;
Sublevel d: 5 orbitals.
The generic representation of these orbitals is done as follows:
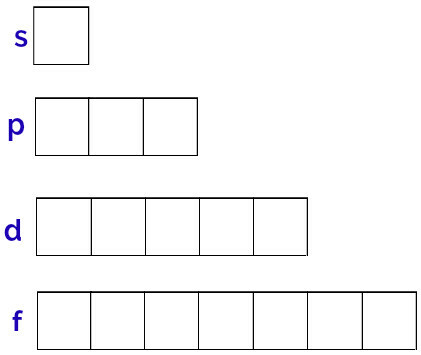
Representation of the orbitals of each sublevel
According to Pauli, an orbital can have a maximum of 2 electrons, with spins (rotating movements) opposite.
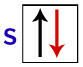
Representation of the s orbital with its electrons
According to Hund, an orbital of a sublevel only receives its second electron when all the other orbitals of that sublevel have already received the first electron.
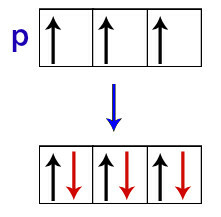
Distribution of electrons in p sublevel orbitals
3O point: electronic distribution
To understand hybridization and the number of bonds that an atom makes, it is essential to carry out the eletronic distribution on the Linus Pauling diagram.
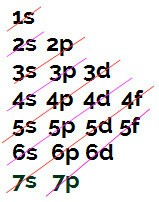
Linus Pauling diagram
Remembering that the maximum number of electrons in each sublevel is:
s = 2 electrons;
p = 6 electrons;
d = 10 electrons;
-
f = 14 electrons.
Do not stop now... There's more after the advertising ;)
After this brief review, we can define now what is hybridization. For this, we will use the chemical element boron (atomic number = 5) as an example.
When we perform electronic boron distribution, we have:
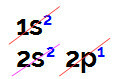
Electronic boron distribution in the Linus Pauling diagram
It is possible to observe in this distribution that boron has 2 electrons in the s sublevel and 1 electron in the p sublevel of valence layer.
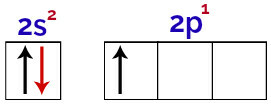
Electrons in the orbitals of the boron valence layer
As boron has 1 incomplete orbital, therefore it should only make one covalent bond, since the number of bonds is always directly related to the number of incomplete orbitals.
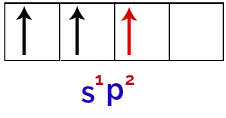
Thus, when the boron atom receives energy from the external environment, its electrons, especially those in the valence shell, become excited. This causes one of the electrons from the s orbital to leave and occupy one of the empty p orbitals, thus resulting in 3 incomplete atomic orbitals, as you can see in the following image:

Representation of the excited state of the boron atom
Finally, there is the union of the incomplete s orbital with the incomplete p orbitals. This union is called hybridization. Since we have the fusion of an s orbital with two p, it is called hybridization sp2.
Representation of hybridized orbitals in the boron atom
In addition to boron, several other chemical elements undergo the phenomenon of hybridization, such as sulfur (S), Xenon (Xe), phosphor (P), carbon (Ç), beryllium (Be).
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is hybridization?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-hibridizacao.htm. Accessed on June 27, 2021.
Binding energy, Chemical reactions, breaking bonds, study of energy variation, enthalpy variation of reactions, energy supply, breakage of the connection of reagents, endothermic process, energy release, energy released in the formation