Polymerization is the name of the chemical process that results in the formation of macromolecules (large molecules) called polymers, through the combination of smaller molecules, the monomers.
the reaction of polymerization it is very common in nature, as we can see in carbohydrates (like starch) and proteins (like casein in milk). It also occurs synthetically, since the vast majority of polymers used by human beings in their daily lives are made artificially.
The first polymer produced from polymerization synthetic was Bakelite, in 1909, by the Belgian chemist Leo Hendrik Baekeland.
In general, for a monomer to be combined with another (whether they are the same or different) in a reaction of polymerization, it is necessary the existence of free valence (chemical bond to be performed) in both monomers.
These valences arise as a result of the breaking of bonds, through the use of catalysts (such as nickel), external conditions such as light and heat, or by the phenomenon of resonance in the structure (shift of electrons).
In the formation of polypropylene (PP polymer), for example, used in household utensils and toys, the pi link (π) in each molecule is broken down as follows:
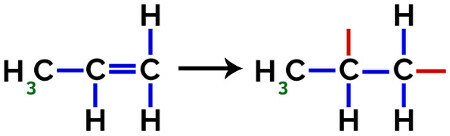
Breakage of pi bond in propylene
Thus, each propylene monomer can bond with two other propylene monomers and form the polymer PP or polypropylene (the prefix poly indicates several monomeric units). The most frequent way to represent a polymer has the monomer between parentheses and, on the outside, the letter n, which indicates several monomers, as we can see in the case of the PP polymer:
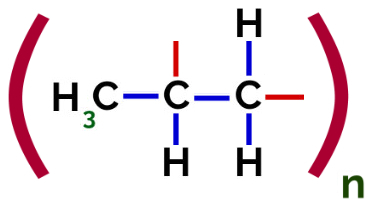
Representation of the PP polymer
the reaction of polymerization can be done in different ways, as we will see below:
a) Reaction of addition polymerization
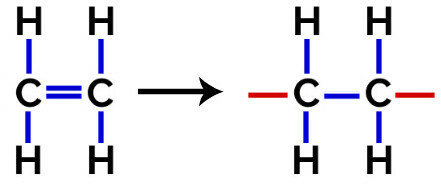
In this polymerization, there is always a break of a pi bond in the monomer, which causes two free valences to appear in the structure, as in the formation of the polyethylene, polymer widely used in pharmaceutical packaging.
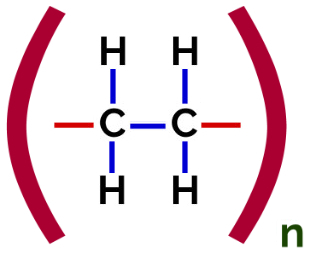
Polyethylene Addition Polymer Structural Formula
At polymerization of polyethylene, molecules of ethylene (ethene) are used as the monomer, which has a pi bond between the two carbon atoms. When this bond is broken, two free valences appear, one on each carbon atom that was involved in the pi bond. The monomers unite exactly in each of these valences, that is, the valence of one is linked to the valence of the other, and so on.
Do not stop now... There's more after the advertising ;)
Polyethylene Formation Equation
b) Addition polymerization reaction 1.4
In this polymerization, the monomers present two alternating double bonds (one pi and one sigma), which favors the phenomenon of resonance (alternating the position of the pi electrons of the pi bond), as in the formation of synthetic rubber (polybutadiene)

Structural formula of polybutadiene
The monomeric unit of this polymer is butadiene, which has two alternating double bonds. With resonance, the structure has a double bond between carbons 2 and 3, and two free valences on carbons 1 and 4. It is precisely in these free valences of carbons 1 and 4 that the monomers combine.

Resonance in butadiene
c) Reaction of condensation polymerization or elimination
It is a reaction of polymerization in which, obligatorily, two monomers (the same or different) simultaneously lose atoms or groups, resulting in two free valences in each one of them. In this way, there is always the elimination of hydrogen from a monomer, which then joins a halogen (F, Cl, Br, I), OH, NH2, or to the CN of the other monomer.
So, in the polymerization by elimination, there is always the formation of water, halogenated acid (HCl, HI, HF, HBr), ammonia (NH3) or hydrocyanic acid (HCN) in addition to the polymer. See, for example, the representation of the formation of polyester, a material used as fabric:

Polyester Formation Equation
Polyester-forming monomers are p-benzenedioic acid and ethane-1,2-diol. We can observe that in this polymerization the elimination of water molecules occurs, since the two monomers have two hydroxyls. In this process, the acid loses the two hydroxyls, and the dialcohol loses only the hydrogen from its hydroxyls:

Polyester Structure
Polyester monomers are joined by the oxygen in the alcohol and the carbon in the carboxyl acid.
By Me. Diogo Lopes Dias
